INTRODUCTION
Cervical cancer is the fourth most frequent cancer in women and seventh in the general population worldwide. In Colombia there were 4,661 new cases of cervical cancer and 1,986 deaths from the disease in 2012, making it the second cause of death from cancer in women1. The social burden created by this disease is significant, particularly considering that 40% of the households in Colombia depend on women for income and sustenance of the children2. From the point of view of the aetiology, it has been found that the disease is preceded by an infection caused by any of the 14 oncogenic types of the human papilloma virus (HPV) [16, 18, 31, 33, 35, 45, 51, 53, 68] defined as “high risk” (hr-HPV) by the International Agency for Research on Cancer (IARC) in 20093.
Following a hr-HPV infection, a small percentage of women develop cervical epithelium abnormalities known as scamous intrepithelial lesions (SIL) in cytology, or also as “intraepithelial neoplasms” (CIN). Low grade cervical intraepithelial neoplasms (CIN1) have a high probability of regression (60-80%) within the first two years after detection and do not require treatment4. However, in 10% of cases, they may persist and progress to high grade neoplasms (CIN2 or CIN3), and 30% of these progress to carcinoma and, consequently, need to be treated4.
Unlike other types of cancer, early diagnosis is possible in cervical cancer, even 10-15 years before it manifests, through the identification of precancerous intraepithelial lesions (SIL), using either cervical cytology or, more recentely, HPV detection.
Cytology-based screening programs started in the 1960s with very good results in developed countries, where they have been well organised, achieving more than 70% coverage. In those countries, mortality rates from cervical cancer have dropped by 50-90%5. However, in middle and low income countries, results in terms of incidence and mortality have not followed the same trend, given that cytology has important limitations associated with coverage and access to health services, poor sample processing, low sensitivity, poor follow-up of positive patients, and high infrastructure and human resource costs(5). In Colombia, 9% of women of screening age have never had a cytology, 12% have had a cytology only once in their lives, and 30% of women fail to collect the result2. It has been described that women do not attend screening programs due to cultural or subjective reasons such as pain, fear, shame of having the sample taken by a male practitioner, or lack of awareness about the disease6.
The implementation of HPV-based screening programs has brought about a reduction in deaths from cervical cancer(3, 5). A systematic review of 40 studies (144,000 women) found that the relative sensitivity of the HPV test vs. traditional cytology for the detection of CIN3+ is 1.46 (95% CI: 1.12-1.91) and relative specificity is 0.95 (95% CI: 0.93-0.97)7. On the other hand, Ronco et al., based on data from four European controlled clinical trials, conclude that HPV testing offers 60-70% more protection against invasive cancer compared with cytology, given its higher sensitivity for the detection of cervical intraepithelial neoplasia8. HPV identification may be accomplished with tests for direct hr-HPV DNA detection DNA viral fragment amplification tests, or viral mRNA identification tests. The first type of test can detect any type of hr-HPV DNA without prior DNA amplification.
The second option uses conventional polymerase chain reaction (PCR) or real-time amplification of a DNA fragment in order to make millions of copies, and the mRNA test identifies overexpression of the E6 and E7 hr-HPV oncoproteins7. Colombia is in the process of gradually replacing cytology with HPV tests for screening4.
HPV testing has the advantage of allowing the patient to collect the sample by herself (self-collected) and delivering it to the screening programs. It has been described that self-collection of samples might increase coverage by 10 to 15% and reduce cervical cancer rates9-11. Self-sampling offers an attractive alternative due to ease and privacy reasons in countries where cultural barriers and program structures limit access to screening12-15. Different types of devices for self-sampling have been designed, such as swabs, brushes, tampons or flushing devices16-18.
A meta-analysis concludes that, regardless of the type of device used (swab, brush, tampon) for sample collection, the sensitivity and specificity of the self-collected samples for detecting HPV infection are similar to those of the samples taken by the clinician19. HPV testing can be done on self-collected vaginal samples, which offers an opportunity to improve screening coverage.
However, the clinical accuracy of HPV testing on self-collected samples is not well known. We assessed whether HPV testing on self-collected samples is equivalent to HPV testing on samples collected by clinicians.
We identified relevant studies through a search in PubMed, Embase and CENTRAL. Studies were eligible if they fulfilled all of the following selection criteria: a self-collected cervical cell sample followed by a sample taken by a clinician; a high risk HPV test done on the self-collected sample (index test). Cervicovaginal lavage is the best studied self-sampling technique, but its main disadvantage relates to the proper handling of fluid specimens which are difficult to transport. Hence the importance of having an option for dry storage and transportation of HPV DNA11,17. This will have a favourable impact on coverage and will create an opportunity for cervical cancer screening in primary care settings20,21.
In Latin America, because of the heterogeneity of its territory, the presence of indigenous populations and remote areas with difficult access to healthcare services, implementing self-sampling is a strategy worth assessing for HPV-based detection of cervical cancer. Concordance between the self-collected sample and the sample obtained by the physician must be determined, as well as whether the quality of viral DNA is preserved in the stored sample until it is analysed. Although some studies have reported HPV DNA stability in a sample during a one-month period22,23, in Colombia there are no studies assessing this topic and it is important to determine whether the test behaves in a similar way in different contexts.
The objective of this study is to assess the usefulness of a new collection device for the identification and preservation of high-risk HPV DNA in self-collected cervicovaginal samples stored dried during 14 days at room temperature.
MATERIALS AND METHODS
Design and population. Diagnostic concordance pilot study of non-pregnant women over 25 years of age attending the cervical pathology and colposcopy units with a result of cervical intraepithelial neoplasia (CIN) grade 1 or more, confirmed on biopsy. Women with a history of total hysterectomy, menstruating or who had used topical vaginal medications within the past 15 days were excluded.
The study was conducted between August 1 and November 30, 2016, in two centres in the city of Bogota: ESIMED clinic, a private institution that serves a population of the contributive insurance regime of the social security system in Colombia, and Engativa Hospital, a public institution that is part of the ESE Northern Integrated Health Service Subnetwork and serves the population covered by the state-subsidised insurance regime. Convenience sampling was used in the universe of patients with cervical dysplasia seen in the participating centres during the study period.
Procedure. The study was designed in two phases: the aim of the first phase was to assess concordance between the results of the dry vaginal samples collected by the patients (self-collection) using the HerSwab® device (Eve Medical, Toronto, ON) and the results of cervical samples taken in fluid medium by the physician for the detection of HPV DNA in patients with cervical dysplasia. The second phase was designed to assess the effectiveness of this device for HPV DNA preservation in self-collected vaginal samples stored dry at room temperature during a 14-day period, compared to a second self-collected sample using the same device and processed on the same initial day.
Phase 1: Candidates to participate in the study received verbal information about the objective, and those who agreed to participate signed the informed consent and completed a survey designed to determine sociodemographic characteristics. Later, the nurse of the cervical pathology unit provided verbal instructions on the self-sampling technique with the help of an illustrated brochure. The physician provided the patients with the HerSwab® device for self-collection of a vaginal sample in the office bathroom, in accordance with the following manufacturer’s instructions: “introduce the device in the vagina, turn the distal end clockwise 5 times to expose the brush and, without removing it from the vagina, turn counterclockwise 5 times to pull the brush back in, then remove the device from the vagina and give it to the nurse”. Patients were then taken to the office where the physician would collect a second sample inserting the Cervi-collect ® device (Abbott) in the endocervix. The device was turned 5 times and then rinsed in the liquid transport medium, in accordance with the manufacturer’s instructions. The sample collected by the physician was always obtained after self-sampling to avoid injuring the vaginal mucosa with the speculum and the resulting risk of contaminating the sample. Once the samples were received in the laboratory, they were processed and analysed on the same day by a trained bacteriologist, in accordance with the manufacturer’s instructions.
Phase 2: A second group of women received verbal information about the objective of the study, and those who agreed to participate signed the informed consent and completed a survey designed to determine sociodemographic characteristics.
Later, they received verbal information from the nurse on the self-sampling technique with the help of an illustrated brochure. The physician gave two HerSwab® devices and asked the patient to take two vaginal samples in accordance with the manufacturer’s instructions, in the same way as they were given to the women who participated in phase 1 of the study. The two samples were received by the nurse, stored dry and transported at room temperature to the laboratory. Once the samples were received in the laboratory, one of them was processed and analysed immediately (time 0), and the second once was stored at room temperature in the same collection device and analysed 14 days later. The bacteriologist who processed the sample on day 14 had no knowledge of the result of the first sample.
Sample processing: Real-time PCR (Real Time, Abbott®) was used to identify HPV DNA in accordance with the manufacturer’s instructions and the standard laboratory procedure. This test is designed for detection of the 14 types of high-risk HPV ([16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68] with genotyping for HPV16 and 18. For DNA extraction, all the samples were prepared using the Abbott m2000sp system (Abbott, Wiesbaden, Germany). Viral DNA detection was defined as positive when the threshold cycle (Tc) achieved was 32 or lower.
Baseline sociodemographic and clinical variables as well as outcome variables were measured. The latter included the result of the self-collected sample and of the sample taken by the clinician, the amount of DNA in the samples taken on day 1 (time 0) processed on the same day and on day 14.
Statistical analysis. Descriptive statistics were used for clinical and sociodemographic characteristics. Means and standard deviations (SD) or medians and ranges were calculated depending on the type of variable, and HPV prevalence was calculated by means of PCR. Moreover, the proportion of positive samples for HPV infection was calculated based on the self-collected and clinician-collected samples processed at time 0 of the two phases of the study.
Overall concordance for the detection of HPV DNA between self-collected samples and samples collected by the physician, as well as Cohen’s kappa coefficient (k) were calculated. Values of k < 0.40 indicate poor concordance, values between 0.41 a 0.75 indicate good agreement, and values of > 0.75 indicate excellent agreement. To determine the ability of preserving viral DNA in the self-collected samples kept in dry storage, mean differences were calculated for related samples in terms of threshold Ct (number of cycles required for each curve to reach a threshold in the fluorescence signal) between dry samples processed at time 0 and at 14 days. Threshold Ct is directly proportional to the amount of DNA present in each sample: the higher the amount of DNA, the lower the number of cycles (Ct) required to reach the threshold. Data were analysed using the SPSS version 19 software. Proportions in the patient preference scale are presented.
Ethical considerations. The study was approved by the Ethics Committee and the Research Committee of USS Engativá (July 22, 2016). The women who participated were asked to sign the informed consent, and the confidentiality of the information was secured.
RESULTS
Overall, 93 women with a biopsy histopathology report were included: 22 with CIN 1, 36 with CIN 2, 31 with CIN 3, and 4 with invasive carcinoma. Patients had been referred for colposcopy due to abnormal cytology: ASCUS (22), ASC-H (n = 11), LSIL (n = 35), HSIL (n = 21) AGC-NOS (n = 2), and negative (n = 2). Mean age was 35.1 years (SD ± 9.16); 63,4% (59/93) of the participants came from a low income bracket and 36.6% (34/93) from a middle income bracket; median age of first intercourse was 17 (range 13-28); median age of first delivery was 19 (range 13-32); median number of deliveries was 2 (range 0-6); and median number of sex partners was 3 (range 1-50).
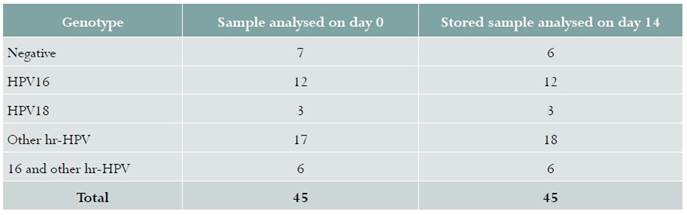
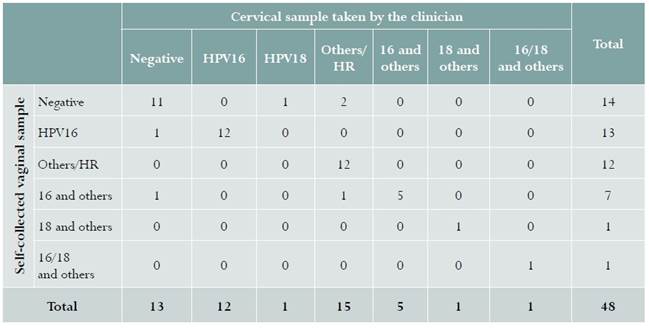
Samples from 48 women were included in phase 1. Infection from hr-HPV in samples taken by the clinician was detected in 35/48 (72.9%) women, compared to 34/48 (70.8%) in self-collected samples, with a kappa coefficient of 0.84 (95% CI: 0.71-0.96). Table 1 shows the different HPV genotypes identified using this technique.
Table 1 HPV phenotype identified in a self-collected sample and a sample collected by the clinician in women with cervical dysplasia in Bogotá, Colombia, 2016
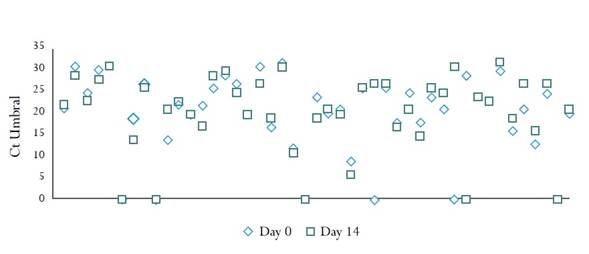
Samples from 45 women were included in phase 2. Figure 1 shows the data for the 45 samples analysed on day 0 and on day 14. The amount of viral DNA detected in the dry sample processed on day 0 (mean Ct 20.11 [SD ± 9.10]) was similar to the one found in the samples processed on day 14 (mean Ct 20.45 [SD ± 8.79]). Mean differences = -0,34 (95% CI -2.29 - 1.61). There were no differences in hr-HPV percent positivity between specimens analysed on the day of collection, 38/45 (84.4%), and the samples analysed 14 days later 39/45 (86.6%) (Table 2). A sample which was negative on day 0 was positive for other hr-HPVs on day 14 (Table 2).
DISCUSSION
Our results show high concordance in self-collected dry vaginal samples for HPV DNA detection when compared with cervical samples taken in fluid medium by the clinician. A prevalence of HPV infection of 72.9% was found in women with cervical dysplasia, slightly higher than the prevalence of 70.8% for self-sampling.
This frequency is greater than the one reported by Baars et al. (53%) in samples collected by the women with the same type of brush. The difference could be explained by the fact that the samples in our study were analysed using PCR, whereas the SPF10-DEIA-LiPA25 system was used by van Baars.20 Our results differ from the systematic review reported by Stewart et al., that found poor concordance in the detection of HPV infection between patient-collected and clinician-collected samples (kappa < 0.6) in three of the studies retrieved in which the vaginal brush was used for self-sampling21.
In our study, the amount of HPV DNA detected in dry self-collected samples using the HerSwab device and stored at room temperature during 2 weeks was the same as detected in samples taken in the same conditions and processed on the same day. These findings are consistent with other studies that report that DNA stability in a dry self-collected sample is preserved for a period of up to two weeks22. Baay et al. reported that the amount and quality of HPV DNA remain stable for up to one month (mean DNA concentration was 45.1 ng /μl, vs. 50.9 ng /μl for samples processed on the same day) without losing the ability to detect high grade intraepithelial lesions23.
Small samples size and the fact that it was conducted in two referral centres in women with dysplastic lesions are limitations of this study because it creates doubt in terms of the possibility of extrapolating our results to the general population where prevalences are lower given that it includes women without preneoplastic lesions. The strengths of this study include the use of DNA detection tests and the fact that the reviewers of the samples taken by the clinician and of the results on day 14 did not know the result of the self-collected samples.
CONCLUSIONS
Our results indicate that vaginal samples taken by the women using the HerSwab® brush are similar in terms of hr-HPV DNA identification to those obtained by the clinician, and that this devise preserves HPV DNA during 14 days if stored dry at room temperature. Studies in the general population are required in order to confirm the effectiveness of the test in screening programs.











 text in
text in