INTRODUCTION
Thyroid diseases are known to be more frequent in women. It has also been described that both hypothyroidism and hyperthyroidism may affect pregnant women1. It is estimated that hypothyroidism occurs in 0.5 to 2.5% of all pregnancies, while thyrotoxicosis is less frequent, with a prevalence ranging between 0.1 and 1%. In a substantial proportion of cases, thyroid disease is associated with immunity disorders2.
The most frequently found antithyroid antibodies in the general population are antithyroperoxidase antibodies (TPOAbs), which are directed against thyroid mitochondrial peroxidase. They are cytotoxic and have been associated with postpartum thyroiditis and psychiatric symptoms. Other detectable antibodies are antithyroglobulin antibodies (TGAbs), and their determination is important in endemic goiter areas and as markers for thyroid cancer3. On the other hand, anti-thyroid stimulating-hormone (TSH) receptor antibodies (TRAb) are heterogenous and classified as having stimulating or blocking activity. They may cause hyper as well as hypothyroidism. A third class of neutral anti-TSH receptor antibodies has been described4.
Environmental risk factors for the development of thyroid autoimmunity include iodine overload and selenium deficiency. Potential immunogenic mechanisms have been proposed in the case of iodine, including the production of proinflammatory cytokines, increased oxidative stress and tissue injury, and higher thyroglobulin antigenic activity5. On the other hand, dietary supplementation with selenium, a micronutrient that participates in biochemical reactions of thyroid hormonogenesis has been found to reduce anti-TPO levels6. However, evidence remains controversial to this date7.
Antithyroid antibodies have been detected in close to 50% of pregnant women with subclinical hypothyroidism and in more than 80% with clinical hypothyroidism8, although some studies show that they may be present in patients with normal TSH and thyroid hormone levels9. On the other hand, the presence of antiperoxidase or antithyroglobulin antibodies has been reported during pregnancy but not accompanied by overt thyroid disease or subclinical hypothyroidism. Reports vary according to the different authors, with figures ranging between 2 and 20%8,10. Moreover, La’ulu and Roberts report ethnic variations in the prevalence of gestational thyroid autoimmunity. Of a total of 3064 serum samples, positive TGAbs were found in 10.6% of Asian patients, 1.8% of black patients, 6.2% of Hispanic women and 6.5% of white patients. As for TPOAbs, they were positive in 12.4% of Asian patients, 4.1% of black women, 11.8% of hispanic women, and 12.3% of white patients11.
Even though subclinical hyperthyroidism has not been associated with obstetric complications, subclinical hypothyroidism and thyroid autoimmunity are a different story2. Some studies have reported the association between the presence of antithyroid antibodies and lower implantation rates, and a higher frequency of miscarriage in invitro fertilization procedures12-14. Other studies have suggested an association between positive thyroid autoimmunity and the presence of obstetric complications such as preterm labour15,16; however, evidence regarding gestational diabetes and pregnancy-related hypertension is controversial17,18. According to the American Thyroid Association (ATA) guidelines8, evidence is not sufficient to recommend universal screening for thyroid autoimmunity during pregnancy. As of today, testing is reserved for patients with risk factors (e.g., a family history, recurrent miscarriage or other associated autoimmune diseases).
There are few regional reports regarding specific reference hormonal ranges for different population types (mestizo, African descendants and indigenous ethnic groups), just like there is a paucity of studies on the prevalence of gestational thyroid autoimmunity or on the association of anti-thyroid antibodies with obstetric complications. Bearing in mind the ethnic variations mentioned in the literature regarding thyroid stimulating hormone ranges as well as the prevalence of antibodies, and given the absence of available data for our population, the objective of this study is to describe the prevalence of thyroid autoimmunity in a hospital-based population of pregnant women in Santa Marta, Magdalena (Colombia), and to perform an exploratory analysis of the detection of TPO and TG antibodies and the presence of obstetric complications such as miscarriage, threatened or actual preterm delivery, and pregnancy-related hypertensive disorders.
MATERIALS AND METHODS
Design and Population. Descriptive cross-sectional study. The base population consisted of pregnant women seen at Centros Hospitalarios del Caribe (CEHOCA), during the period between August 1 and October 31, 2017. This private healthcare institution provides primary care services and is also a referral center for high complexity patients (adults, children and pregnant women). It receives patients coming from the Department of Magdalena, on the Caribbean coast of Colombia.
The study enrolled patients over 18 years of age with singleton pregnancies coming to the emergency room (at any gestational age) or who went into spontaneous labour (at term), or came for elective cesarean section, and voluntarily agreed to participate in the research. Patients with multiple pregnancy, known past or present thyroid disease (hypothyroidism or hyperthyroidism), or with other pre-existing maternal morbidities were excluded. To estimate the sample size, a base population of 900 patients, an expected prevalence of gestational thyroid autoimmunity of 10%18, a 95% confidence level and a margin of error of 5% were used. Sample size: 120 subjects. Convenience sampling was used.
Procedure. All the candidates to participate in the study were explained the objectives of the research and invited to participate; once they agreed, they were asked to sign the informed consent. After training the clinical staff on the data collection modality, they were given printed forms to enter the data. The process of sampling, delivering the specimens to the laboratory and treating data confidentially was supported by the institutional staff.
Samples of peripheral venous blood were collected and preserved in vials. Samples that were not processed within the next 48 hours were refrigerated at a temperature of 2-8 degrees Celsius (oC), while samples processed over the next 30 days were stored at -20oC. Free thyroxine (T4), free triiodothyronine (T3) and TSH serum levels were determined in each sample in order to detect patients with unknown pre-existing thyroid disease. TSH ultrasensitive testing was performed using a third generation technique: chemiluminescent microparticle immunoassay (CMIA). Likewise, free T4 and T3 levels were determined using the same methodology. Antithyroglobulin (TGA) and antiperoxidase (TPO) antithyroid antibodies were also determined using chemiluminescent microparticle testing for IgG antibody detection. In order to avoid bias, the staff of the laboratory in charge of processing the samples were blinded at all times to patient clinical data and to the basis of the study.
The 2017 ATA guidelines (Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and Postpartum) were considered for hormonal ranges8. These guidelines suggest an upper limit in the reference range of 4 milliunits per liter (mU/L) for TSH, starting in the first trimester. Hypothyroidism was defined as a TSH higher than 4 mU/L with a lower free T4 level, or TSH ≥10 mU/L and normal free T4 level. Hyperthyroidism was defined as TSH suppression (≤ 0,1 mU/L), with high triiodothyronine (T3) or free thyroxine (T4) values8. Collected data were tabulated for analysis. Peer review was applied in order to ensure the quality of the information.
Measured variables. Measured variables included maternal age, parity, gestational age, body mass index15, socioeconomic bracket, education, marital status, personal and family medical history, TSH and free T3 and T4 levels, TPOAb and TGAb levels, presence of subclinical hypothyroidism and antibody levels in euthyroid and hypothyroid pregnant women, and presence of pregnancy complications (miscarriage, threatened or actual preterm delivery, pregnancy-related hypertension and intra-uterine growth restriction).
Statistical analysis. Data were analyzed using the Epi-Info 7® software package19. Continuous variables were expressed as means and standard deviations, or medians and ranges according to normality. In pregnant women with no complications, 3rd and 97th percentiles were determined for the TSH and free T3 and T4 values. Categorical variables were expressed as proportions. Prevalence was calculated as the number of women with positive TG or TPO antibodies/number of surveyed women, and was categorized by positive antibody type and thyroid function (normal or hypothyroidism). To determine the association with obstetrical complications, the groups of women with positive and negative antibodies were compared using odds ratio (OR).
Ethical considerations. The study protocol was approved by the Ethics Committee of Universidad del Magdalena and authorized by Centros Hospitalarios del Caribe (CEHOCA). All the participants signed the informed consent. Confidentiality of the information was ensured. The researchers made the commitment to inform the treating physicians and the patients in the event thyroid disease was diagnosed, in order to ensure treatment and follow-up.
RESULTS
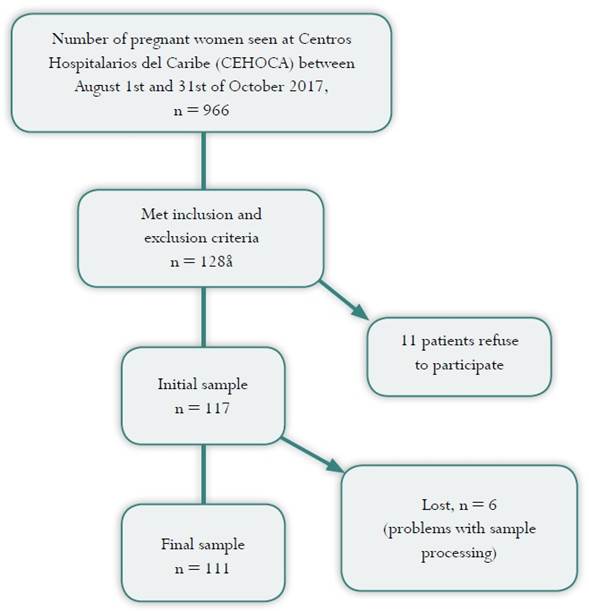
A total of 966 pregnant women were seen at Centros Hospitalarios del Caribe (CEHOCA) in the obstetric outpatient clinic or admitted for vaginal term delivery or elective cesarean section between August 1 and October 31, 2017. Of this universe, 117 patients met the study inclusion and exclusion criteria. During the course of the study, 6 participants were excluded due to issues with specimen processing or incomplete data, so the final sample consisted of 111 pregnant women (Figure 1).
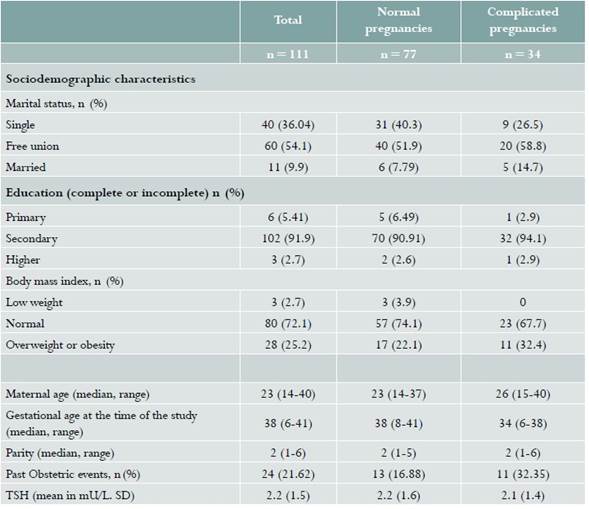
The median age in the sample analyzed was 23 years, with a range between 14 and 40. Gestational age at the time of the test ranged between 6 and 41 weeks, spanning the three trimesters of pregnancy. Median parity was 2. Based on the data taken from the clinical records, most of the women had normal weight, followed in frequency by the overweight and obesity groups. The majority of the women reported being in a de facto marital relationship, and the most frequent level of schooling was secondary education (complete or incomplete) (see Table 1). Of the 111 pregnant women, 77 did not have reported pregnancy-related complications, while miscarriage was diagnosed in 11 cases, 6 had threatened or actual preterm delivery, and 17 were diagnosed with hypertensive disorders of pregnancy.
Table 1 Baseline characteristics of a population of pregnant women of Clínica CEHOCA, Santa Marta (Colombia), studied between August 1 and October 31, 2017
Mean TSH in the 77 patients with uncomplicated pregnancies was 2.2 mU/L, with a standard deviation (SD) of ± 1.56. The mean values for free T3 and T4 were 2.59 pg/ml (SD ± 0.42) and 0.85 ng/dl (SD ± 0.12), respectively. Table 2 shows the distribution of TSH and thyroid hormone values.
Table 2 Distribution of TSH and thyroid hormone values in uncomplicated cases studied in a population of pregnant women of Clínica CEHOCA, Santa Marta (Colombia), between August 1 and October 31, 2017
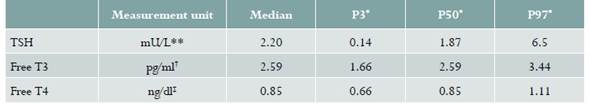
* Percentiles
** milli units per liter
† picogram por milliliter
‡ nanogram per deciliter
Thyroid autoimmunity: 5 patients (4.5%) had positive TPOAbs, 14 patients (12.61%) positive TGAbs, while the two types of antibodies were found to be positive in 3 patients. The frequency of subclinical hypothyroidism (TSH ≥ 4 mU/L and ≤ 10 mU/L, with normal free T3 and T4 levels) was 9.9%. Using a lower cut-off value (TSH ≥ 3 mU/L and ≤ 10 mU/L), the frequency of subclinical hypothyroidism was 19.82%.
The frequency of positive thyroid autoimmunity was 13.5% in euthyroid patients, whereas in patients with subclinical hypothyroidism (TSH between 3 and 10 mU/L) the frequency was 18.9%. Hormonal and antibody determinations are described in detail in Table 3.
Table 3 Hormonal and antibody determinations in a population of pregnant women of Clínica CEHOCA, Santa Marta (Colombia), between August 1 and October 31, 2017
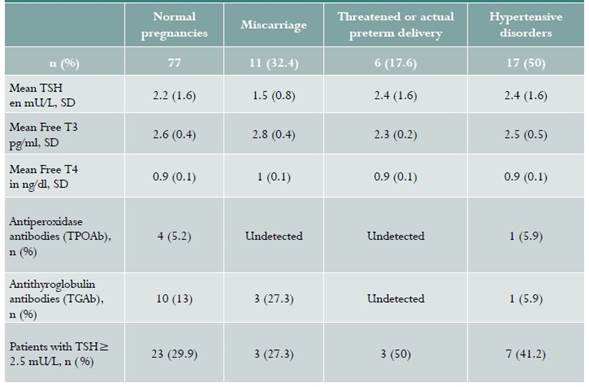
Regarding the association between autoimmunity and obstetric complications, the frequency of thyroid autoimmunity was 14.29% (11/77) in the women with normal pregnancy, and 14.71% (5/34) in women who presented with obstetric complications (OR = 1.03; 95% CI: 0,32-3,24; p value 0.95). The frequency of thyroid autoimmunity was 27.3% (3/11) in patients who miscarried, and 11.8% (2/17) in patients with hypertensive disorders. No autoimmunity was detected in patients with threatened or actual preterm delivery, and one of the 2 patients diagnosed with intrauterine growth restriction was positive for antiperoxidase antibodies and had a high TSH value.
DISCUSSION
This study is an approach to the determination of thyroid autoimmunity prevalence during pregnancy in our setting. Prevalence of positive TPOAb and TGAb was 4.5% and 12.6%, respectively, and 2.7% for the two positive antibodies. The prevalence of subclinical hypothyroidism ranged between 9.9 and 19.82%, depending on the cut-off values used. A 13.5% frequency of thyroid autoimmunity was found in euthyroid women, while in patients with hypothyroidism it was 18.9%. The following values were found in the study population: TSH, 2.18 mU/L (SD ± 1,5); T3, 2.57 pg/ml (SD ± 0,42); and T4, 0.86 ng/dl (SD ± 0,11). No association was found between the presence of autoimmunity and obstetric complications.
Regarding the presence of TGAb and TPOAb, although the prevalence reported in the literature for antiperoxidase antibodies in the general population is higher20, our study found higher positivity for antithyroglobulin antibodies, probably explained on the basis of the sensitivity of the analytical methodology and the possibility of ethnic variations.
Regarding the prevalence of subclinical hypothyroidism, our results are consistent with some reports of frequency ranges of 3.5-18%, depending on the TSH values used in the definition21-23. However, the frequency of subclinical hypothyroidism reported in other studies is much lower, again underscoring the need for local population characterizations1,24,25.
Regarding TSH and free T4 and T3, mean values found in our study are within the range of expected values8. Of the 111 patients surveyed, 22 (19.82%) had TSH levels ≥ 3 mU/L, the upper limit of the normal reference range mentioned in the old ATA guidelines(20), and all of them were in their third trimester of gestation.
In our exploratory analysis, no association was found between the presence of autoimmunity and obstetric complications, unlike what other authors have found in larger populations26-28.
Given that convenience sample was used in this study, the potential risk of selection bias and the fact that the findings cannot be extrapolated to the general population are weaknesses. On the other hand, the methodology used to estimate TSH, free T4 and T3, and antibody levels, may be considered a strength of this study.
CONCLUSIONS
The prevalence of TPOAb and TGAb positivity was 4.5% and 12.61%, respectively. No association was found between the presence of autoimmunity and obstetric complications. Population studies are needed in order to establish normality ranges in the general population.











 text in
text in