INTRODUCTION
Gestational trophoblastic disease (GTD) is an infrequent, biologically heterogenous condition, consisting of differentiation abnormalities or trophoblastic epithelium proliferation1,2. Those abnormalities are classified as villous or non-villous lesions and cover a broad spectrum of disease conditions, ranging from benign lesions to malignant metastatic and non-metastatic neoplasms. Non-villous GTD conditions include placental over-reaction and placental site nodule, both of them benign1. Villous GTDs include benign conditions: molar pregnancy, partial hydatidiform mole and complete hydatidiform mole. Malignant gestational trophoblastic neoplasms include choriocarcinoma, placental site trophoblastic tumour (PSTT), epithelioid trophoblastic tumour (ETT) and invasive mole1,3.
The placental site trophoblastic tumour (PSTT) is a rare subtype with the potential of local invasion and metastasis. It accounts for nearly 1-2% of GTDs1, with an incidence of 1 in 100,000 pregnancies4. Age of onset varies, and in a review of 108 cases, 80% of the women were under 40 years of age4. It typically follows term pregnancies, but they may occur months or even years after any obstetric event2. Initial symptoms include abnormal uterine bleeding or amenorrhea, and in some unusual cases women present with clinical conditions such as nephrotic syndrome or virilisation4.
PSTT arises from the intermediate trophoblast and, consequently, is associated with low levels of betahuman chorionic gonadotropin (beta-hCG)2.
Due to relative resistance to chemotherapy, the treatment of choice for PSTT is hysterectomy when the disease is localised and confined to the uterus in patients who no longer want to preserve their fertility1-3. In premenopausal women, oophorectomy is not indicated in the absence of pelvic involvement3. Given that it is a rare condition, no optimal chemotherapy regimen is known. The most widely used regimens are EMA-CO (etoposide, methotrexate, actinomycin, cyclophosphamide and vincristine), and EMA-EP (etoposide, methotrexate, folinic acid and cisplatin)1,3.
Mortality from this entity has been described as ranging between 6.5 and 20% (4). Poor prognostic factors include distant metastases, intergenesic interval of more than 2 years, mitotic index higher than 5/10, tumour size, deep myometrial involvement, vascular invasion, and severe necrosis. In these cases, it is recommended to combine surgery with chemotherapy with multiple agents1-4.
On rare occasions, PSTT may be associated with renal paraneoplastic syndromes such as nephrotic syndrome, as has been described in case reports2,4,5-13. Nephrotic syndrome is a clinical manifestation diagnosed on the basis of oedema associated with proteinuria of more than 3 g in 24 hours, and albumin of less than 3 g/dL. It is called nephroticnephritic syndrome when associated with arterial hypertension and haematuria14. GTD has been associated with different histopathological diagnoses, including membranous glomerulopathy, membranous proliferative glomerulonephritis, minimal change disease, and glomerular endotheliosis characteristic of preeclampsia15-19, which means that there is no specific associated glomerulopathy. The pathogenesis of paraneoplastic glomerulonephritis is mediated by humoral factors or by the immune response to the malignant cells20,21, and it may sometimes precede the diagnosis of the neoplastic disease22.
IgA nephropathy is the most frequent primary glomerulopathy, although it may have secondary aetiologies, mostly chronic inflammatory conditions and neoplasms. The main associations described include kidney, gastrointestinal and respiratory tract cancers(23). In the majority of cases, the course is asymptomatic, indolent and slowly progressing, marked by the presence of microscopic haematuria (equal or greater than 3 red blood cells per high magnification field in urinary sediment); close to 5% of cases may present as nephrotic syndrome and up to 40% may progress to advanced chronic renal disease(23). In primary cases, treatment consists of blocking the renin-angiotensin-aldosterone system and the use of steroids; in cases of paraneoplastic glomerulopathy, effective cancer treatment leads to remission of the glomerular lesion20-22. For this reason, the use of immunosuppression therapy could be reasonable in cases that do not improve or in cases of life-threatening clinical presentation, such as rapidly progressing glomerulonephritis or severe nephrotic syndrome14,20.
Due to limited information regarding the renal compromise in patients with GTD and the association with PSTT, the objective of this study is to report the case of a patient with placental site throphoblastic tumour who presented with nephrotic syndrome secondary to IgA nephropathy, and who recovered from the renal compromise following total hysterectomy and medical management, with no need for additional immunosuppression.
CASE PRESENTATION
A 23-year-old patient, primigravida, referred in January 2017 from a health centre located in the rural area of the Department of Cundinamarca in central Colombia. She presented to the centre at 15 weeks with amenorrhea, periorbital and lower limb oedema associated with “coloured foamy urine”, with no other urinary symptoms or fever. In December 2016, because of worsening oedema, the patient attended the prenatal care clinic where findings included blood pressure of 164/110 mm/Hg, a 7 kg weight increase, enlarged uterus and anasarca, prompting referral to San Ignacio University Hospital, a level IV institution in Bogota that serves a population affiliated to the contributive as well as the state-subsidised systems.
Findings during the physical examination included the following: weight 69 kg; height, 156 cm; blood pressure, 180/100 mm/Hg; heart rate: 100 beats per minute; respiratory rate: 16 per minute; oxygen saturation: 94% in ambient air; periorbital oedema; pustulous lesions in the forehead and malar region of the face. Cardiopulmonary auscultation was normal and, on abdominal palpation, the uterus was enlarged and no foetal heart rate was recorded on Doppler examination; there was grade III foveal oedema of the limbs; and the neurological examination did not reveal any abnormalities. Paraclinical tests were ordered based on the diagnostic impression of nephrotic syndrome, with the following results: haemoglobin, 12.7 g/dL; leukocytes, 10100/uL; platelets, 209000/uL; creatinine, 0.53 mg/dL; urinalysis with proteinuria (500 mg/dL); microscopic haematuria (50 red blood cells/high power field), and leukocyturia (30 leukocytes/high power field). Additional tests showed the following results: serum albumin, 1.5 g/dL; total cholesterol, 163,2 mg/dL; high-density cholesterol (HDL), 37 mg/dL; triglycerides, 183.3 mg/dL; proteins in 24-hour urine, 23 g; and normal renal ultrasound. Nephrotic-nephritic syndrome was confirmed, considering IgA nephropathy versus lupus nephritis as differential diagnoses. Consequently, additional tests were requested, with C3 and C4 complement being normal, and ELISA results being negative for hepatitis B surface antigen, hepatitis C antibodies and human immunodeficiency virus. This ruled out the possibility of post-infectious glomerulonephritis which typically results in reduced complement levels. Given the suspicion of lupus nephritis, antinuclear antibodies were requested and came back positive at 1/320 with mottled pattern; however, extractable antibodies (antiRo, antiLa, antiSm and Anti RNP) and double strand DNA antibodies were negative, lowering the possibility of lupus nephritis. A renal biopsy with complete histopathological study was ordered. Moreover, serum beta-human chorionic gonadotropin (b-hCG) was high at 834,40 mUI/mL. Obstetric ultrasound showed a uterus 75.3x59.9x62.2 mm in size, 25 mm cervix with closed internal and external os and no gestational sac; a heterogenous image 40.6 mm in size with cystic pattern of irregular contours was observed in the endometrial cavity; adnexa were not visualised. The diagnostic impression of a molar pregnancy or a uterine sarcoma prompted an abdominal and pelvic contrast computed tomography (CT) scan which showed an enlarged uterus of heterogenous density with no adnexal masses.
A transvaginal follow-up ultrasound was performed 48 hours after admission, showing an anechoic uterine image 11.1 mm in size, possibly in relation with an irregular hypotonic gestational sac, with poor choriodecidual reaction. There was a 12% reduction of beta-hCG down to 729.11 mIU/ Ml leading to a diagnosis of incomplete miscarriage. The patient was taken to dilation and curettage (D&C) with the following findings: 11 cm uterus, uterine cavity of irregular surface, and abundant firmly adhered tissue. The patient remained in the hospital and was started on medical treatment for the nephrotic syndrome with intravenous (IV) furosemide 1 mg/kg as initial bolus and a maintenance dose of 10 mg IV every 6 hours, 10 mg of amlodipine daily and 150 micrograms of clonidine every 8 hours, leading to improvement of arterial pressure, although anasarca persisted.
The kidney biopsy showed 18 glomeruli, without global or segmental sclerosis, mesangiocapillary glomerulonephritis, and IgA deposits on immunofluorescence (Figures 1 and 2), leading to the conclusion of IgA nephropathy. Given the severity of the clinical picture, a multidisciplinary team decided to initiate treatment with 3 doses of methylprednisolone, 500 mg daily, followed by prednisolone, 50 mg daily, together with enalapril, 20 mg every 12 hours, furosemide as described previously plus spironolactone, 25 mg every 24 hours, and hydrochlorothiazide, 25 mg daily, in order to improve the nephrotic syndrome.
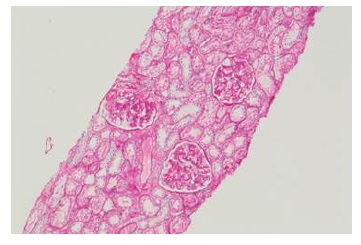
Figure 1 Renal biopsy, light microscopy (PAS, 10X): glomeruli without global or segmental sclerosis, mesangiocapillary glomerulonephritis and early intracapillary proliferation mediated by predominantly mesangial immune complexes.
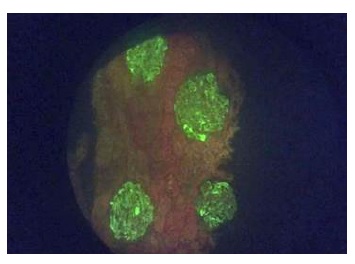
Figure 2 Renal biopsy, direct immunoflourescence (20X). Granular mesangial immune complex deposits for IgG (++), granular mesangial IgA (+++), granular mesangial IgM (++), granular mesangial and membrane C3 (+), C1q (-), albumin (-), granular mesangial fibrinogen (++), granular mesangial kappa (++), granular mesangial lambda (+++)
One week later, the pathology report of the D&C confirmed a placental site trophoblastic tumour (Figure 3). The gynaecological oncologist assessed the patient and asked for a follow-up beta-hCG which came back at 668.82 mIU/mL; extension studies were negative for metastatic disease. Given the type of tumour and the high probability of resistance to chemotherapy, the patient was taken to total abdominal hysterectomy plus bilateral salpingectomy through laparotomy. The final pathology report documented a uterine endometrial cavity replaced by a friable tumour mass 8 cm in its longest dimension, with areas of necrotic and haemorrhagic appearance which infiltrated the entire myometrium into the serosa. The microscopic findings were consistent with a placental site trophoblastic tumour of aggressive behaviour due to its size and extensive involvement of the myometrial wall and a focal clear cell component (Figure 3). Immunohistochemistry markers showed tumour cells that were positive for CKAE1/3, inhibin, CD10 and beta-hCG focally, and negative for p63 and p53. The cell proliferation index (Ki67) was 15%.
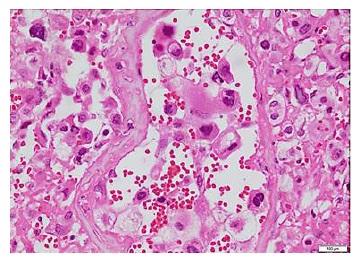
Figure 3 Endometrium. Haemotoxylin and Eosin staining, 4x. Placental site trophoblastic tumour. Fibrinoid necrosis in vessel wall and intraluminal tumour cells
In view of the histopathological findings, the aetiology of the renal compromise was reconsidered and IgA nephropathy was interpreted as a paraneoplastic condition. Consequently, steroids were discontinued and diuretic management was continued with oral furosemide 40 mg every 12 hours, enalapril 20 mg every 12 hours, and atorvastatin 40 mg daily. The oedema resolved and weight dropped to 57 kg, leading to discontinuation of spironolactone and hydrochlorothiazide. At that point, the patient was discharged and continued on outpatient management.
Three weeks postoperatively, beta-hCG dropped to 76,69 mIU/mL, and at 3 months it was negative (1.2 mIU/mL). At the 3-month follow-up visit, proteinuria had dropped by more than 50% (594 mg/24 h), serum albumin had recovered (3.6 g/L) and creatinine remained normal (0.46 mg/dl). The clinical oncologist assessed the patient and adjuvant chemotherapy was proposed based on the final pathology report, the presence of localized FIGO stage I disease treated surgically, lowering of beta-hCG, but with a high risk of recurrence due to high mitotic index, myometrial infiltration by the tumour and histological type, but the patient refused. After one year of follow-up, the patient does not have a recurrence and the nephrotic syndrome has resolved.
Ethical considerations. Information confidentiality was ensured. The patient data publication protocols of the institution (San Ignacio University Hospital) were followed, and the approval of the ethics and research committee was obtained. Patient right to privacy was respected and informed consent was obtained for presenting the case. Photographs were taken by the authors.
DISCUSSION
PSTT is an infrequent condition, with only a little more than 300 cases described in the literature3, 4). They are reported not to secrete beta-hCG but in fact it is not that the hormone is not produced but that the vast majority of these tumours (> 90%) synthesise it at a much lower level when compared to all other types of gestational trophoblastic neoplasms, probably due to the fact that they arise from the intermediate trophoblast; it usually does not exceed 1000 mIU/mL, as presented in this case2. More frequently, patients have had prior obstetric events, although the case presented here was the first pregnancy. On rare occasions, clinical manifestations in other systems have been described in PSTT, including haemoptysis, ascites, virilisation and nephrotic syndrome4. In the case described here, the patient presented with nephrotic-nephritic syndrome, and kidney histopathology showed IgA nephropathy. Several paraneoplastic syndromes have been described in association with gynaecological tumours, with neurological, ophthalmological, dermatologic, rheumatological, endocrine, haematological and renal involvement22. In the renal system, the most frequent clinical presentation is nephrotic syndrome and the most common histological finding is membranous glomerulopathy, found in relation with solid tumours such as cervical and endometrial carcinomas, and choriocarcinoma22. Of the 17 cases of renal compromise associated with GTD, 9 occurred in patients with PSTT10,11,16-18 and a renal biopsy was performed in 4 of them10,11,16, with varying histological findings: one case of thrombotic microangiopathy10, one case of lupus nephritis11, and two cases with prominent intra-luminal occlusive eosinophilic deposits, with IgM deposits16. IgA nephropathy, which was documented in this patient, was not found in any of the cases.
IgA nephropathy is usually a primary condition and, occasionally, it develops into nephrotic syndrome, although it may be associated with neoplasms. The review did not find cases of PSTT associated IgA4,6-13,16-19,22. Renal biopsy in pregnant patients is guided by gestational age, the glomerular syndrome present and potential change of therapy, always taking into consideration the risks for the mother and the foetus. Reports in the literature refer to a frequency of renal biopsy complications of 7% during pregnancy and 1% in the postpartum period; consequently, decision should be individualised14,24. Of the total number of cases of GTD-related renal involvement17, renal biopsy was performed in 12 patients, with no complications6-13,16-19. In this case presentation there were no complications associated with the renal biopsy.
Treatment. The cornerstone in the treatment of IgA nephropathy in the general population is the use of renin-angiotensin-aldosterone system blockers (angiotensin converting enzyme [ACE] inhibitors or aldosterone receptor antagonists [ARA II]), together with salt intake restriction to less than 5 g per day and tight blood pressure control (< 125/75 mm/Hg). In patients with concomitant nephrotic syndrome, adding steroids at a dose of 0.5-1 mg/kg/day for 4-6 weeks is recommended14,23. In cases with no nephrotic syndrome or renal function decline, 3 to 6 months must be allowed in order to assess response to ACEi or ARAII treatment; if proteinuria remains above 1 g, prednisolone 0.5-1 mg/kg/day for 6 months must be added. During pregnancy, the use of ACE inhibitors and ARA II is contraindicated and treatment is therefore based on salt intake restriction. Monitoring is based on creatinine and proteinuria in 24-hour urine, and remission is considered to have been achieved when proteinuria comes down to less than 500 mg/24 h14,23.
Treatment of the underlying disease is recommended in cases of paraneoplastic glomerulonephritis20-22, with the expected result of nephropathy remission. In the case presented here, when the histopathology confirmed the diagnosis of PSTT, it was decided to discontinue immunosuppression and maintain treatment with the ACE inhibitor in order to manage residual proteinuria and blood pressure. In some case reports, steroids were used for 6 months; however, these medications have a profile of significant side effects, arterial hypertension, hyperglicaemia and infections, mandating their rational use(14).
In the cases of PSTT found in the review, all patients were taken to hysterectomy and some received chemotherapy, and there were two fatal outcomes secondary to infection16,18. In the patients who overcame the acute event, renal compromise resolved without the need for immunosuppression therapy6-13. In the case presented here, renal involvement resolved after surgical removal of the tumour and, although the patient refused chemotherapy, she is still relapse-free.
CONCLUSION
There are only a few descriptions of IgA nephropathy associated with PSTT in the literature. Prognosis of renal involvement secondary to a neoplastic pathology is usually good, considering that once the neoplastic focus is removed, proteinuria seems to resolve. Tight clinical and paraclinical follow-up is crucial.











 text in
text in 


