INTRODUCTION
The vaginal microbioma is dominated by Lactobacillus spp. and members of the Clostridiaceae, Bacteriodetes y Actinomycetes families 1. Among the Lactobacillus genus, the most frequently isolated species are Lactobacillus gasseri, Lactobacillus crispatus, Lactobacillus jensenii and Lactobacillus iners. These lactic acid-producing bacteria protect the urogenital tract from pathogenic microorganisms through, a) specific epithelial adhesion, blocking their settlement; b) production of antimicrobial compounds which maintain a pH < 4.5 and have a microbicidal effect; c) co-aggregation with pathogens, enhancing their microbicidal effect 2. Thus, they play a critical role in the inhibition of bacterial and fungal infections of the urogenital tract 3. When the microbiome is disrupted, fungal infections or vaginal flora derangement known as bacterial vaginosis (BV) may be triggered and manifest in the form of inflammation (vaginitis) and symptoms (itching, foul smell, etc.), or without any symptoms 4. In the former case, vaginitis may result from the proliferation of endogenous agents, such as Candida spp., or exogenous sexually transmitted agents, such as Trichomonas vaginalis. BV results from an imbalance in the vaginal ecosystem, involving a displacement of Lactobacillus spp. by proliferating anaerobic and Gardenella-type bacteria 5.
During pregnancy, Candida infection results from the rise in sexual hormones like progesterone, which induces reduced local cellular response in the vaginal epithelium, mediated by its immunomodulatory action on T lymphocytes and polymorphonuclear cells, and estrogens, which increase glycogen in the vaginal tissue, creating a carbon-rich environment for yeast development 6. In particular, studies by Leli et al. show that pregnant women tend to have more frequent asymptomatic colonization by Candida spp. than their non-pregnant counterparts (46.5% vs. 16.0%) 7. Likewise, this type of infection has been associated with an increased risk of chorioamnionitis, vulvar vestibulitis syndrome, and premature delivery during the third trimester of gestation 8,9. In the case of neonatal infection, the birth canal is the main source of Candida albicans, unlike other species associated to a large degree with horizontal transmission 10. The diagnosis of Candida spp. vaginitis is based on the finding of yeasts plus hyphae or pseudohyphae in the test with 10% KOH, and more than 5 leukocytes per field in the Gram stain, as indicator of vaginal inflammatory response (VIR). Apart from selective yeast isolation in Sabouraud agar, chromogenic agars enable differentiation of C. albicans5,11,12, present in 90% of cases, from other species such as C. glabrata, C. parapsilosis and C. tropicalis. Culture is considered the gold standard.
On the other hand, pregnant women with asymptomatic infections caused by the Trichomonas vaginalis protozoon may transmit them vertically to the neonate during childbirth, giving rise to neonatal genitourinary infection or pneumonia 9-11. Diagnosis involves direct microscopic examination of the discharge, Giemsa staining, and protozoon culture; the latter is the reference method for diagnosis, with 98% sensitivity 5.
BV is another condition associated with adverse outcomes in pregnancy, including a higher risk of premature delivery, premature rupture of membranes, and even miscarriage 13. In this case, an increase in the number of anaerobic or facultative germs would precipitate these outcomes 14. Diagnosis may be made using Amsel’s clinical and microbiological criteria 15, and analyzing the bacterial morphotypes present in the Gram stain, in accordance with Nugent’s criteria, considered the gold standard 5.
Consequently, asymptomatic infections may predispose to ascending vaginal tract colonization, fetal membrane infiltration and amniotic cavity invasion, with subsequent fetal damage caused by infectious processes which are among the most frequent causes of maternal morbidity and perinatal morbidity and mortality 16-19.
Little is known in our setting about the magnitude and implications of asymptomatic infections caused by these potentially pathogenic agents in pregnant women in the third trimester of gestation. Data regarding the frequency of colonization and perinatal and maternal outcomes in women with asymptomatic infection could help to determine whether individual interventions in these women, or population-wide programs, are required.
This study sought to address the question about frequency and, therefore, its objective was to determine the prevalence of potentially pathogenic agents in the vaginal exudate of a sample of asymptomatic pregnant women in the Department of Atlantico, Colombia.
MATERIALS AND METHODS
Design and population
Descriptive cross-sectional study carried out in pregnant women at 35 to 37 weeks of normal gestation, with no signs or symptoms of lower genital tract infection, seen during the prenatal care program between October 2014 and May 2015 in the city of Barranquilla, in the Caribbean coast of Colombia. The study was conducted in a Level I private healthcare institution that provides services to patients of the subsidized and contributive regimes of the Colombian social security system in health. Women who had received antimicrobial treatment within the past 30 days, with genital bleeding at the time of the exam, mental disability, immunosuppression or treatment with immunosuppressants, were excluded. Sampling was consecutive of women who agreed to participate in the study.
Procedure
Participants were recruited by the gynecologist of the research team, who did the selection during the prenatal follow-up visit. If they met the inclusion criteria and agreed to participate, the objective of the study was explained and were asked to sign the informed consent. After the informed consent was obtained, the patients were administered a data collection tool previously designed in Excel, which included sociodemographic variables and obstetrical and gynecological history; privacy and confidentiality of the information were protected at all times. The gynecologist then proceeded to take a sample from the vaginal cul-de-sac exudate for microbiological testing, including a first brush sample in saline solution for fresh testing, Gram stain smear, pH measurement and amine test; and a second brush sample in Stuart transport medium, with no charcoal, for culture and May-Grunwald
Giemsa staining
Processing was carried out by a microbiologist trained in the protocols established by the Microbiology Laboratory of Universidad Metropolitana de Barranquilla.
The diagnosis of Candida spp. vaginitis was based on the detection of yeasts plus hyphae or pseudohyphae in the direct mount with 10% KOH, in the Gram stain and in the isolation in the CHROMagar Candida (Becton-Dickinson) culture medium. Final identification was made using the semiautomated API 20C AUX (bioMérieux) method. The diagnosis of T. vaginalis vaginitis was based on microscopic observation of trophozoites in the fresh mount and the May-Grunwald Giemsa staining 20.
Nugent’s 21 and Amsel’s 15 criteria were implemented concomitantly in the diagnosis of BV. With Nugent’s criteria used as the reference method, the presence of bacterial morphotypes by immersion field under Gram staining were quantified (Lactobacillus spp., Gardnerella-Prevotella and Mobiluncus); BV was considered to be present when the score was between 7 and 10, with intermediate flora between 4 and 6, and normal microbial flora between 0 and 3. Applying Amsel’s microbiological criteria, BV was diagnosed when the criteria and the following parameters were met: presence in the Gram stain of scaly epithelial cells covered by short Gram-variable rods (guide cells), pH ≥ 4.5, and positive amine test.
A significant VIR as a criterion for vaginitis was considered as the finding of more than 5 leukocytes per field (400 X magnification) in the Gram stain, or a count of more than 10 in fresh mounting.
Measured variables
The following variables were measured: maternal age, gestational age, age of initiation of sexual and obstetric life, number of sexual partners, marital status, place of origin, schooling, history of urinary tract infections (UTIs), antibiotic treatment, asymptomatic bacteriuria, history of miscarriages, preterm labor, and sexually transmitted diseases.
Statistical analysis
The Statgraphics version 16 software package was used for analyzing the study variables. Quantitative variables were expressed as means and standard deviation; qualitative variables were expressed as proportions. Overall prevalence: number of women with Candida, T. vaginalis or BV / number of women examined. Specific prevalence: number of women with infection / number of assessed women at risk.
Ethical considerations
The study was approved by the Ethics Committee of Universidad Metropolitana de Barranquilla, preserving the principles of confidentiality and privacy of all the pregnant women. At all times, the microbiologist reported the results to the gynecologist, in accordance with the established methodology. The gynecologist was responsible for informing the participants and taking the necessary steps for their treatment and follow-up under the prenatal care program.
RESULTS
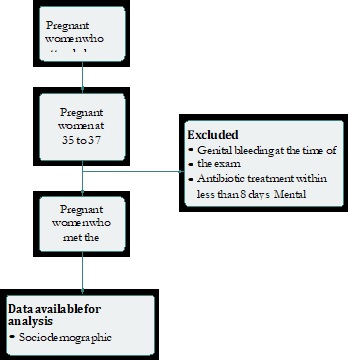
During the observation period, 300 asymptomatic patients considered potential candidates for the study were selected. Of them, 260 met the inclusion and exclusion criteria and, of these, 226 (86%) agreed to participate in the study (Figure 1).
Of the 226 pregnant women who participated in the study, 53% lived in the city of Barranquilla, 20% in Soledad and 27% in other townships of the department. Mean age was 24.5 years (± 6.0 SD). In terms of schooling, 10.6% had only primary education, 75.2% had completed secondary education, and 14.2% had higher education.
In terms of sexual activity, 77.4% of the women had started their sex life between 15 and 19 years of age, 14.2% after 19 years of age, and 8.4% between 10 and 14 years of age. At the time of the study, 48% had had more than one sexual partner, and mean maternal age at the time of having their first child was 20 years, ranging from a minimum of 13 and a maximum of 38. There was a history of miscarriages in 25% (n= 57) of the women, preterm labor in 12.3% (n= 28), UTIs in 40.2% (n= 91) ITU, and 2(1%) had a history of a sexually transmitted disease. The general prevalence vaginal microbiota disruptions was 24.8% (56/226). Of these, 55.4% (31/56) were due to vaginitis and 44.6% (25/56) to vaginosis. By type of pathogen, the prevalence of asymptomatic Candida infection was 13.3% (30/226), and of T. vaginalis infection was 0.4% (1/226). In terms of yeast-related vaginitis, 70.0% (n = 21) was due to C. albicans and 30.0% (n = 9) to non-albicans Candida.
Regarding the prevalence of BV, when Nugent’s criteria were applied in order to detect bacterial morphotypes associated with abnormalities, BV was found in 8.0% (18/226) and intermediate flora in 3.1% (7/226), a category which, although not a diagnosis of vaginosis, is considered a disruption of the normal vaginal flora. Using the Amsel test, the prevalence was 7.1% (n = 16). Significant VIR was found in 56.7% (17/31) of cases of C. albicans or T. vaginalis or T. vaginalis colonization.
DISCUSSION
Our results found a general prevalence of potentially pathogenic microbiological agents in 24.8% (56/226) of asymptomatic pregnant women at 35-37 weeks of gestation; Candida vaginitis was the most prevalent, found in 13.3% (30/226) of cases, and vaginitis due to T. vaginalis was found only in 0.4% of cases (1/226). The prevalence of BV was 8%.
Our results are similar to those reported by Farr et al. in asymptomatic pregnant women in Viena, Austria, who reported a frequency of C. albicans of 13.5% 22) and are lower than those reported by Touzon et al.23 in asymptomatic pregnant women in whom culture for Candida spp. and T. vaginalis was performed together with Nugent’s test for BV, reporting a 19% prevalence of BV, 1% T. vaginalis, and 19.5% Candida spp. They are also lower than the prevalence of Candida spp. colonization reported in Italy by Leli et al. who reported a 35% prevalence in asymptomatic pregnant women 13, and by Duque et al. in Medellín, Colombia, who reported a prevalence of 25.6% of Candida spp. in 162 asymptomatic pregnant women 24. Apart from the study by Touzon et al. mentioned previously, we did not find any reports of T. vaginalis detection in asymptomatic pregnant women.
Regarding the prevalence of BV, our data are similar to those reported by Tolosa et al. in a multi-center study conducted in asymptomatic pregnant women at 18-35 weeks of gestation, showing a prevalence of vaginosis of 9%; our results are higher than those reported in Ireland (5.9%) and the United States (5.8%), and lower than those reported for Myanmar (15.6%) and Zimbabwe (24.4%), with prevalences lower than those reported by Campos in Brazil (26.2%) (25), in pregnant women at 14 to 24 weeks of gestation, or those reported by Menguistie in Ethiopia (26), who reported a prevalence of 15.9% in asymptomatic pregnant women, although with no information regarding the gestational age at which the study was conducted.
A strength of this study is the use of the gold standard for the microbiological diagnosis of infection caused by Cándida spp., T. vaginalis and BV in asymptomatic pregnant women. As for limitations, this study did not include detection of Streptococcus agalactiae, and the type of sampling may have resulted in selection biases.
CONCLUSIONS
Substantial colonization of the lower genital tract by potentially pathogenic germs is found in pregnant women between 35 and 37 weeks of gestation. Further studies are required to determine the benefit of population screening in terms of avoiding poor maternal and perinatal outcomes, as well as the resulting overcosts.











 text in
text in