Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Psiquiatría
Print version ISSN 0034-7450
rev.colomb.psiquiatr. vol.40 suppl.1 Bogotá Oct. 2011
Artículos originales
Subsyndromal Depressive Symptoms in Bipolar II Disorder: a Community Mental Health Services Cohort Study (SIN-DEPRES)
Síntomas depresivos subsintomáticos en trastorno bipolar II: un estudio de cohorte de servicios de salud comunitarios
Rosario de Arce1
Miguel Ángel Jiménez-Arriero2
José Luís Rodríguez-Calvin3
José María Ruiz-Aguado4
Silvia Zaragoza-Domingo5
Silvia Cobaleda6
Eduard Vieta7
Grupo SIN-DePReS
1Bipolar Disorder Unit. Hospital Universitario Puerta de Hierro. Madrid. Spain.
2Psychiatry Department, Hospital Universitario 12 de Octubre. University Complutense. CIBERSAM. Madrid. Spain.
3Psychiatry Unit. Hospital Fuenlabrada. Fuenlabrada (Madrid). Spain.
4Centro de Salud Mental Lakuabizkarra. Vitoria (País Vasco). Spain.
5PSYNCRO. Neuropsychological Research Organization, S.L. Barcelona. Spain.
6Neurosciences Area. Medical Department. GlaxoSmithKline S.A. Tres Cantos (Madrid). Spain.
7Bipolar Disorder Program. Institut Clínic de Neurociencies. Hospital Clinic, IDIBAPS, University of Barcelona, CIBERSAM, Barcelona. Spain, for the SIN-DEPRES Group.
Conflict of interest
Dr R. Arce has received research grants, and served as consultant, advisor or speaker for the following companies: AstraZeneca, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Janssen-Cilag, Novartis, Bristol, Pfizer Inc, Sanofi-Aventis and UCB Pharma.
Dr Vieta has received research grants and served as consultant, advisor or speaker for the following companies: AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Forest Research Institute, Glaxo-Smith-Kline, Janssen-Cilag, Jazz, Lundbeck, Novartis, Organon, Otsuka, Pfizer Inc, Sanofi-Aventis, Servier and UBC, and research funding from the Spanish Ministry of Health, the Spanish Ministry of Science and Education, the Stanley Medical Research Institute and the 7th Framework Program of the European Union.
Dr Jimenez Arriero has received research grants and served as consultant, advisor or speaker for the following companies: Almirall, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Janssen-Cilag, Lundbeck, Novartis, Pfizer Inc, Sanofi-Aventis, Servier and research funding from the Spanish Ministry of Health.
J.L Rodríguez-Calvin and J.M Ruiz-Aguado have not conflicts of interest.
S. Zaragoza is an employee of the contract research organization, PSYNCRO, Neuropsychological Research Organization, S.L., contracted by GSK.
S. Cobaleda is an employee of GlaxoSmithKline, S.A. The sponsor
GSK contributed to the design, conduct, analysis and interpretation of this study. However the final authority on the interpretation of the results was given to the first author (Dr. R. Arce).
Corresponding author
Eduard Vieta
Hospital Clinic & CIBERSAM
Villarroel, 170 08036 Barcelona, España.
evieta@clinic.ub.es
http://www.bipolarclinic.org
Recibido para evaluación: 23 de junio del 2011. Aceptado para publicación: 31 de julio del 2011
Abstract
Objectives: The aim of this study was to assess the prevalence and the impact of subclinical depressive symptoms (SDS) on the functional outcome of bipolar II (BD) outpatients in remission. Methods: Cross-sectional and prospective 16-week study of a cohort of 739 euthymic BD patients included by 94 investigators in Spain. Clinical stability was assessed at baseline and week 16 with the Clinical Global Impression scale for BD (CGI-BP-M), depressive symptoms at baseline with the Hamilton Depression Rating Scale (HDRS), the Montgomery-Asberg Scale (MADRS) and the self-applied Center for Epidemiologic Studies-Depression Scale (CES-D). Functional status was evaluated with the Social and Occupational Functioning Assessment Scale (SOFAS) and Social Adaptation Self-evaluation Scale (SASS). Results: The sample of type II BD was composed by 202 patients. SDS were detected in 21.3% of patients (95% IC =15.9 to 27.6) at baseline. In apparently symptom-free patients, the incidence of SDS after 16 weeks was 29% (MADRS >7). At baseline, SDS patients compared to non-SDS presented poorer social-occupational performance (SOFAS mean difference -13.3, 95% CI from -17.1 to -9.5) and poorer social adjustment (SASS mean difference -4.3, 95% CI from -7.0 to -1.7). Depressive symptoms were inversely related to functional status and social adjustment: MADRS-SOFAS correlation coefficients r = -0.55 (p<0.0001) and MADRS-SASS correlation coefficients r = -0.43 (p<0.0001). The self-applied questionnaire identified additional cases with depressive symptoms at baseline, showing a SDS-Total prevalence of 51% identified by any method. A MADRS score 5 showed 0.75 sensitivity and 0.69 specificity in the detection of cases with possible SDS based on self-reported results as gold standard. Conclusions: Depressive symptoms in apparently remitted type II BD outpatients are common and as frequent as in other BD subtypes. These subclinical symptoms result in adverse occupational outcome and social maladjustment. MADRS and self-applied questionnaires during follow-up visits may provide important information about type II BD patients’ mood status and functionality.
Key words: Bipolar II, depressive symptoms, subsyndromal.
Resumen
Objetivos: Evaluar la prevalencia y el impacto de los síntomas depresivos subclínicos (SDS) en el resultado funcional de pacientes externos de bipolaridad II (TB) en remisión. Métodos: Estudio transversal y prospectivo, de 16 semanas de duración, de una cohorte de 739 pacientes eutímicos de TB incluidos por 94 investigadores en España. La estabilidad clínica se evaluó, en la línea base y en la semana 16, con la Escala Impresión Global Clínica para TB (CGI-BP-M); los síntomas depresivos, en la línea base, con la Escala de Calificación de la Depresión de Hamilton (HDRS), la Escala Montgomery-Asberg (MADRS) y la Escala Autoaplicada para la Depresión del Centro de Estudios Epidemiológicos (CES-D). El estado funcional se evaluó con la Escala de Evaluación del Funcionamiento Social y Ocupacional (SOFAS), y la Escala de Autoevaluación de la Adaptación Social (SASS). Resultados: La muestra de TB tipo II estuvo compuesta de 202 pacientes. Se detectaron SDS en 21,3% de los pacientes (95% IC = 15,9 a 27,6) en la línea base. En pacientes que aparentemente no presentaban síntomas, la incidencia de SDS después de 16 semanas era de un 29% (MADRS>7). En la línea base, los pacientes SDS, en comparación con los no SDS, demostraban un desempeño social-ocupacional más pobre (diferencia media SOFAS -13,3, 95% IC de -17,1 a -9,5) y un ajuste social más pobre (diferencia media SASS -4,3, 95% IC de -7,0 a -1,7). Los síntomas depresivos estaban relacionados inversamente con el estado funcional y el ajuste social: coeficientes de correlación MADRS-SOFAS r = -0,55 (p<0,0001) y coeficientes de correlación MADRS-SASS r = -0,43 (p<0,0001). El cuestionario autoaplicado identificó casos adicionales con síntomas depresivos en la línea base, y mostró una prevalencia total de SDS de 51% identificada por cualquier método. Un puntaje MADRS ≥ 5 mostró una sensibilidad de 0,75 y una especificidad de 0,69 en la detección de casos con posible SDS, basándose en los resultados autoreportados como el estándar de oro. Conclusiones: Los síntomas depresivos en pacientes externos de TB de tipo II aparentemente en remisión son comunes y son tan frecuentes como para los demás subtipos de TB. Estos síntomas subclínicos tienen resultados ocupacionales adversos así como inadaptación social. La MADRS y los cuestionarios autoaplicados durante las visitas de seguimiento pueden ofrecer información importante acerca del estado de ánimo y la funcionalidad del paciente de TB tipo II.
Palabras clave: Transtorno bipolar, síntomas depresivos.
Introduction
Bipolar disorder (BD) is a common, chronic, serious condition affecting approximately 0.8% (type I BD) and 0.5% (type II BD) of the adult population (1,2), although higher prevalence rates have been described in Europe (3.3%) (3). Several studies have documented the persistence of substantial depressive morbidity among patients diagnosed with unipolar or bipolar depression receiving pharmacological treatment. Figures close to 44.9% have been advanced in patients who suffered bipolar depression and 43.3% in unipolar depression (4,5). Many patients diagnosed with BD, despite receiving appropriate treatment and follow-up, may spend up to a third of the year suffering from depressive symptoms (6).
This predominantly depressive nature of BD is now accepted following results of important prospective cohort studies (7-11). Presence of residual symptoms has also been documented to be clearly related to the natural course of the condition: not only to the duration of the disease course but also to the number of recurrences, factors that have important implications for the prevention of relapses (12-15).
Besides this significant role in relation to relapse prediction, the association of residual symptoms with adverse functional impact on patient’s life has also been described (16,17); patients with subclinical depressive symptoms present 3 to 6 times more functional impairment than those who do not have these symptoms (i.e. in various domains such as work, housework, relationships with relatives and friends) (15).
Therefore, the importance of identifying and appropriately treating these symptoms is widely acknowledged, so that patients can obtain complete disease remission and thus their clinical outcomes in the long-term is improved (14).
Type II BD is a serious recurrent condition, with chronic depressive features and course that are much more serious than previously believed (18,19). Consequently, it is important to describe the frequency and outcome of such chronic subclinical symptoms specifically related to type II BD patients.
A wider study of which this publication represents a patient subset was conducted in our setting with the primary objective of obtaining a cross-sectional estimation of the subclinical depressive symptoms (SDS) present among BD symptomatic stable patients cared for in spanish community mental health services (20). The present study aims to report the prevalence and 16-week incidence of depressive symptoms in subsample of type II BD patients.
The present study (SIN DEPRES-type II-BD) also aimed to ascertain the most suitable methods for detecting subsyndromal depressive symptoms in type II BD patients, that would be applicable in clinical practice, thus enabling clinicians to detect prevalent cases in order to put in place appropriate therapeutic strategies.
Materials and Methods
This is a cross-sectional, prospective, 16-week epidemiological study of a cohort of outpatients with clinically stable bipolar disorder. The study was conducted in 88 Spanish centers which enrolled a sample of consecutive outpatients attending Community-based Mental Health Services and private clinics.
Subjects
Patients over 18 years of age with a well-established diagnosis of bipolar II disorder according to DSM-IV-TR criteria (21,22), who had remained clinically stable for at least the last month were recruitet. Clinical stability was defined as a score of “normal” or “minimal” severity in the evaluation of the depression and mania ratings of the Modified Clinical Global Impressions scale for Bipolar Disorder (CGI-BP-M) (23,24). Patients had to have suffered at least one acute episode in the last five years prior to inclusion in the study. Patients were excluded if no reliable information was available at the centre, or if they presented with acute depression, mania, hypomania or mixed symptoms at inclusion, if they suffered from some other serious psychiatric condition, current drug addiction, other conditions affecting the central nervous system, organic brain disease or had suffered any cranio-cerebral trauma, dementia, or an uncontrolled serious medical condition or illness which could produce secondary depression (e.g. hypothyroidism). We also excluded patients who had suffered a single acute episode of bipolar disorder and those patients participating in clinical trials. The present report shows the results corresponding to type II BD patients.
At each centre, healthy volunteer subjects were also selected from clinics other than the psychiatric department to become a control group (HS). These were no members of a BD patient’s family or staff from the psychiatric department. Patient inclusion was consecutive at each centre and the voluntary subjects were the last recruited cohort. The study was conducted from April 2006 to March 2007.
The study was approved by the Independent Ethics Committee of one of the participating centers, Hospital Clinic de Barcelona (25,26). All participants were informed about the study and provided written informed consent prior to inclusion.
Procedure
The study objective and procedures were explained to all subjects. After obtaining their written informed consent, the study data were obtained by means of a clinical interview and psychiatric examination. The interview obtained information about sociodemographic and clinical data (including history of psychiatric disorders in first degree relatives), treatments received and also the degree of treatment compliance and satisfaction with this. The presence of depressive symptoms, the patient’s social-occupational status and functional performance in different aspects of his/her life were also assessed.
Participants, except healthy control subjects, were re-assessed at a second visit after 16 weeks (± 4 weeks) regarding the intensity of depressive symptoms; clinical worsening occurring during the study period, important life events that occurred and which could have affected the patient´s mood and the use of healthcare resources were also recorded.
Clinical assessment
In order to determine the stability of the condition at baseline visit, all patients were evaluated with the Modified Clinical Global Impression Scale for Bipolar Disorder (CGI-BPM) (23,24) ; the 17-item Hamilton Depression Rating Scale (HDRS-17) (27,28)was also administered to record and score any present depressive symptoms and their severity at the time patients were included in the study. Depressive symptoms were both evaluated at enrollment and end of the study using the Mont-gomery-Asberg Depression Rating Scale (MADRS)(29,30).
The patient group with depressive symptoms was defined according to the baseline result in the HDRS17 scale (total score). Prevalence of depressive symptoms was thus defined as the percentage of patients who obtained a total score indicating “mild depression” (score 7 to 17) on the HDRS-17 scale. The subclinical depression status of the patients, divided into two groups (subclinical depressive symptoms - SDS, or non-SDS), was defined by the scoreon this scale.
A description of the depressive symptoms referred by subjects themselves was obtained as supplementary information. The Centre for Epidemiological Studies Depression Scale (CES-D)(31,32), a widely used instrument to detect cases of depression in the general population (33) was likewise administered. The frequency, but not the intensity, of depressive symptoms reported by the subject in the week before the visit was also evaluated.
Evaluation of functional status
In order to evaluate the impact of the condition on the patient’s social-occupational life, the Social and Occupational Functioning Assessment (SOFAS) (21) was used.
The impact of the condition on the patient’s social life was evaluated by means of the Social Adaptation Self-evaluation Scale (SASS) (34-37). This is a self-applied scale used in depression studies to measure social behavior and motivation, revealing the patient’s perception of his/her functional status according to different roles and functional areas.
Data analysis
The sample size calculation was described elsewhere (20) in order to determine the prevalence of the SDS among BD patients, reported to be around 44,9% (4). Control group size was calculated to detect differences on the HDRS-17 scale total score between healthy subjects and BD subjects based on previous results reported (38).
As a first step, type II BD patients were compared to type I BD patients with regards to socio-demographic and clinical features to ascertain the external validity of the study results.
An estimation of the prevalence of SDS among type II BD patients and the figure was also adjusted by the population of the different Spanish participating regions.
The mean total score on the HDRS-17 scale for type II BD patients was compared with the result obtained from the healthy control group using a Student’s T-test, excluding the item addressed to score the awareness of the condition.
The prevalence result of SDS among type II BD patients based on the HDRS-17 scale, was combined with the results obtained with the self-applied CES-D questionnaire, considering a score of over 15 as a possible case of SDS. With this information, the total prevalence of baseline SDS in the sample (i.e. total SDS) was defined; the SDS rate detected by either one of the two instruments was also recorded.
The degree of consistency between the two instruments, HDRS-17 and CES-D was analyzed by a consistency analysis using Kappa statistic.
In order to qualitatively characterize the patients with a positive result for SDS in only the self-applied test (CES-D), these patients were assessed with regards their socio-demographic and clinical variables and a comparison was made with results from the CES-D and SASS scales with patients who obtained a positive result with both instruments using a Chi-2 or Student’s T-test, as appropriate.
We also analyzed whether patients with baseline SDS, according to HDRS-17, presented a greater risk of relapse during follow-up, assessing the difference in the risk of presenting a new episode among patients according to their baseline SDS status.
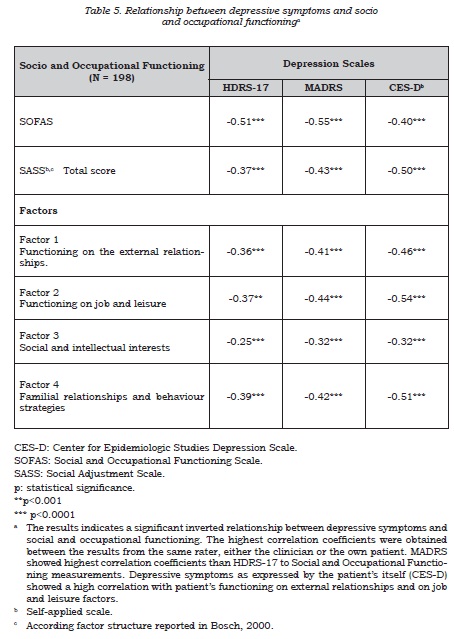
The relationship between subclinical depressive symptoms and social-occupational functioning was studied by calculating correlation coefficients.
The incidence of SDS was determined in type II BD patients apparently in remission at baseline assessment, who had no recurrences during the follow-up period. The incidence of SDS was assessed by two methods: (i) the proportion of patients presenting with clinically significant increases on the MADRS scale (an increase of at least 50% on the score in this scale relative to the baseline resulting in a final score of more than 7) and (ii) the proportion of patients who obtained a score of more than 7 on this scale at the end of follow-up (39).
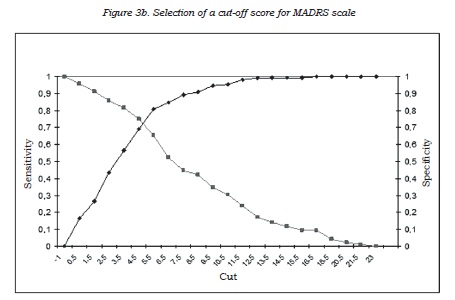
To identify the best clinical method for detecting depressive symptoms at follow up with any of the two scales used (HDRS-17 versus MADRS), which not only showed the best correlation with the result on the self-reported CES-D scale but also best correlated with the result of the self-reported SASS was selected. This was achieved by calculating Pearson and partial correlations (40). Once the scale had been selected according to these two criteria, the best cut-off point by means of ROC curves was determined, considering the result of the self-applied depression questionnaire as the “gold standard” (or external validation variable).
All the statistical tests were interpreted considering a significance level of 5%. No corrections were made for multiple comparisons and all differences were considered in order to increase the possibility of findingdifferences (41). A non parametric approach was considered for the analysis of parameters based on time estimations, or for those measurements with a non-normal distribution. The SAS statistical package, release 8.2 (SAS. Institute Inc., 2005), was used for all statistical analyses.
Results
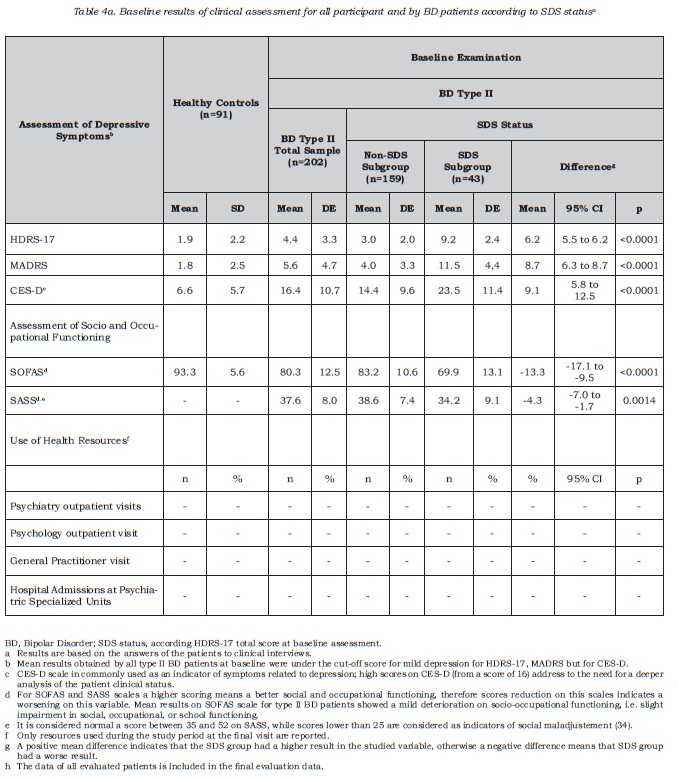
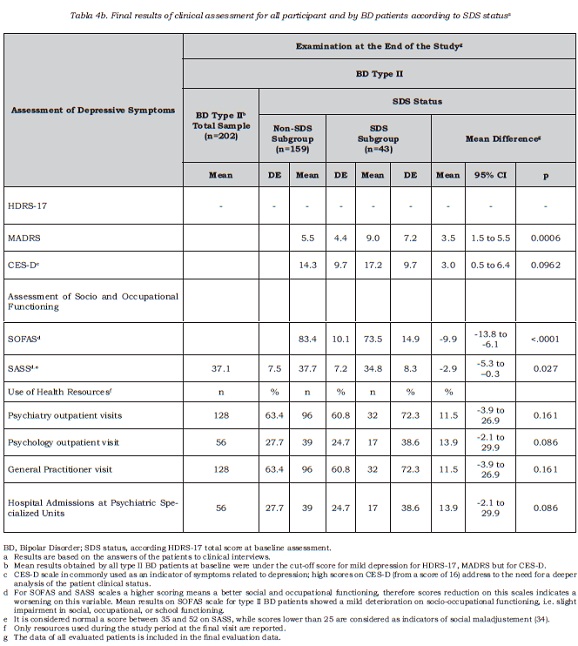
The study included a sample of 761 BD I and II patients , 739 of whom were included in the analysis. Those excluded (n = 22) were deviations from the protocol due to failing to meet the stability criteria (CGI-BP-M), having presented less than one acute episode in the last 5 years or presence of another medical condition such as hypothyroidism. The group of healthy volunteers comprised 91 subjects. Table 1 describes the sociodemographic and clinical characteristics of all the participants. The most common type of bipolar disorder was type I (n = 537, 72.7%), followed by type II (n = 202, 27.3%). At baseline, type II patients were older than type I BD patients, and within this group, there was also a higher proportion of women and people living independently compared to type I BD. With regards to clinical variables, type II patients were also older than type I patients at the age of first episode and on average suffered more episodes per year. Furthermore, there was a higher rate of these with a past history of depressive episodes among BD type II patients, and the last depressive episode was also closer to baseline visit, compared to type I BD patients (see Table 1 for results regarding all variables). As expected, most type II BD patients suffered at least one depressive episode in the past (98%, n = 198), and this was the desease polarity for the most recent episode for the majority of patients in this subsample (70.8%, n = 143).
Subclinical or mild depression detected by interview
Among type II BD patients, subclinical depressive symptoms were detected in 43 out of 202 cases, 21.3% (95% IC =15.9 to 27.6). Only one patient obtained a score indicating high desease severity (score higher than 17 on HAMD-17), who has been not included in the SDS prevalence figure. The adjustment to population of Spanish participating regions did not provide additional information, resulting in similar percentages (21.3%; 95% IC = 21.2% to 21.3%). Table 2 shows the sociodemographic and clinical characteristics of the type II BD sample according to SDS status (SDS or non-SDS). Both groups, SDS and non-SDS, were comparable regarding sociodemographic and clinical variables, with the exception of the duration of clinical stability. For this variable, the results suggested that SDS patients, at baseline assessment, had spent less time clinically stable in comparison with non-SDS patients. For the whole BD type II sample, clinical stability lasted nearly one year; for SDS patients it was less than one year and this resulted in a mean difference of about 7.8 months of shorter stability period (DE 28.9) compared to non-SDS patients. No significant association was observed between the polarity of the most recent episode and the presence of subclinical depression, that is, SDS status.
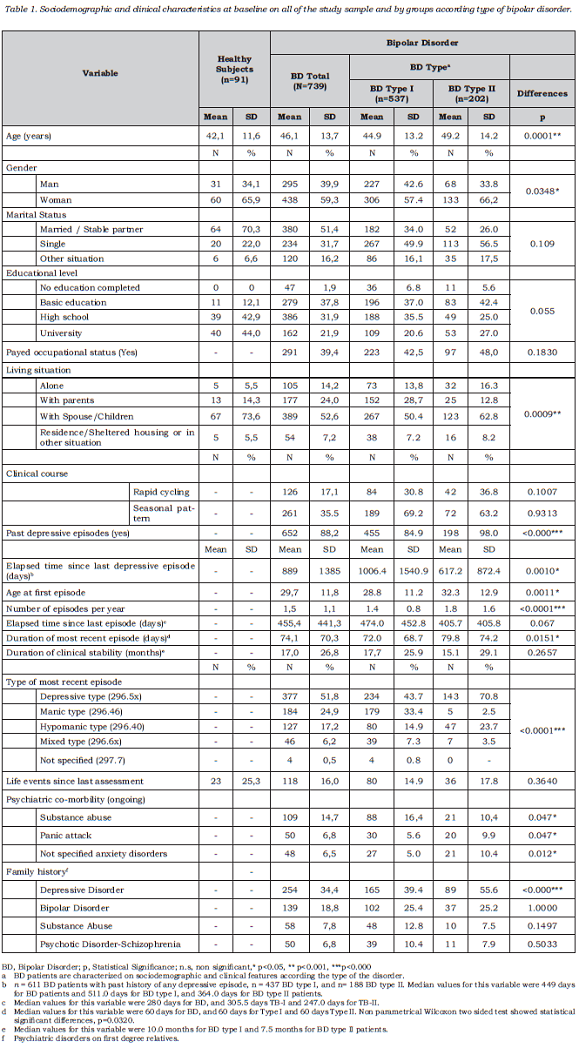
Figure 1 shows the mean severity obtained for each item on the HDRS-17 scale in type II BD patients according to SDS status; item severity was greater for SDS than for non-SDS patients, the differences being particularly marked for those items related to insomnia and psychic anxiety depressed mood and impact on work and activities.
Table 4 shows the results obtained from clinical examination at both study visits (Baseline and End of study).
Validity of the measurement of depressive symptoms
The difference found between type II BD and control subjects (CS) groups on the HDRS-17 scale score showed a mean of 2.7 points (95% CI = 1.9 to 3.4): type II BD patients obtained higher scores than healthy control subjects (table 2); when specific items were analyzed, patients with type II BD obtained higher scores than the healthy control subjects in the majority of items except for the following items where both groups showed similar results: (item 4) insomnia early , (item 6) insomnia late, (item 12) gastrointestinal symptoms, (item 15) hypochondria and (item 17) weight loss.
CS participants were comparable to type II BD patients, exhibiting similar sociodemographic variables except that CS being slightly younger (mean difference of 7 years, SD 12.4, 95% CI = 3.7 to 10.4, p<.0001) and having a higher level of education (44.4% of the CS had university degrees, versus 27.9% in the type II BD group) (table 1). Therefore, HDRS-17 resulted a valid measure of depressive symptoms among asymptomatic subjects, specifically for type II BD patients.
Self-reported depressive symptoms and prevalence of total SDS
The percentage of patients with scores showing mild depression in the CES-D scale was similar to the percentage of patients detected by clinical interview, 15.4% (31/202, 95% CI = 10.7 to 21.1). However, and not coinciding with the results obtained with the HAMD-17 scale, an additional group composed of 59 patients were identified as having moderate-severe depression in this self-applied questionnaire (i.e. a score of 21 or more). Thus, and added to the other group, a total of 45.5% of subjects had depressive symptoms (92 cases out of 202) according to the self-applied questionnaire. The two detecting methods (i.e. HDRS-17 and CES-D) were concordant, in relation to a positive SDS status, in 33 out of 202 type II BD patients; 59 new additional cases of possible subclinical depression were identified with the self-applied questionnaire, being non-SDS according to clinical interview. In this way, very few patients obtained a normal score on CES-D when being identified as SDS patient by HAMD-17 (5%, 11 out 202). The resulting kappa consistency coefficient was 0.27 (95% CI = 0.15 to 0.39, p<0.0001), thus showing fair consistency (42). Therefore, with the two assessment tools, 51% (103 out 202) of the type II BD patients studied presented some degree of the depressive symptoms as identified by either method (SDS-Total).
Table 3 shows the results obtained following the comparison between patients who were only detected by the self-reported questionnaire (n=59, 57.2% of Total-SDS) with those detected by both methods (n=33, 32.0% of Total-SDS) regarding self-reported measurements. As regards sociodemographic and clinical features, patients detected only by self-reported methods were slightly older than those detected by both methods; patients showing depressive symptoms by clinical interview had a mean age of 43.2 years, (SD 13.1), whereas patients detected only by self-reported methods had a mean age of 51 years (SD 12.6)(mean difference among groups of7.8 years, SD 12.8, 95% CI = 2.313.3, p < 0.0064). Age at the first episode was similar for both groups, in their early early thirties (p= 0.34) but patients detected only by the self-reported method showed a longer stability period; differences showed statistical significance with a mean of 22 months, (SD 49.7) (median of 8 months, 95% CI 9.1 to 34.9) in patients detected only by self-applied questionnaire versus a mean of 8.9 months, SD 9.7 (median 5, 95% IC 5.5 to 12.3) of stability in patients detected by both methods; mean difference between groups was near 13 months (95% CI -4.3 to 30.5, Wilcoxon test, p= 0.0422). Results of clinical scales at baseline showed lower intensity of symptoms in those patients detected only by self-applied tests in comparison with those detected by both methods: a mean on the HDRS-17 scale score of 3.8, (SD 1.7) versus 9.5, (SD 2.6) (p<0.0001) respectively and a mean on the MADRS scale of 5.7, (SD 4.0) versus 12.1, (SD 12.0) (p<0.0001) respectively. A better socio-occupational functioning indicated by a mean score on SOFAS of 82.2, (SD 12.2) versus 73.1, (SD 4.5) (p=0.0002) was also shown.
When comparing CES-D and SASS scales with its factors, patients detected only by the self-applied questionnaire showed a slightly lower frequency of depressive symptoms by self-applied scale than those detected by both methods, with a lesser irritability component and hopeless feelings, as they obtained a significant lower score on factor 3 of CESD (34). Some differences were also found regarding social adjustment, i.e. patients detected only by the self-applied questionnaire showed a better social adjustment as measured by SASS scale; specifically, a better functioning either in familiar and external relationships and in behavior strategies (factors 1 and 4 of SASS, respectively) as well (43).
Detection of SDS in clinical practice and methodological aspects
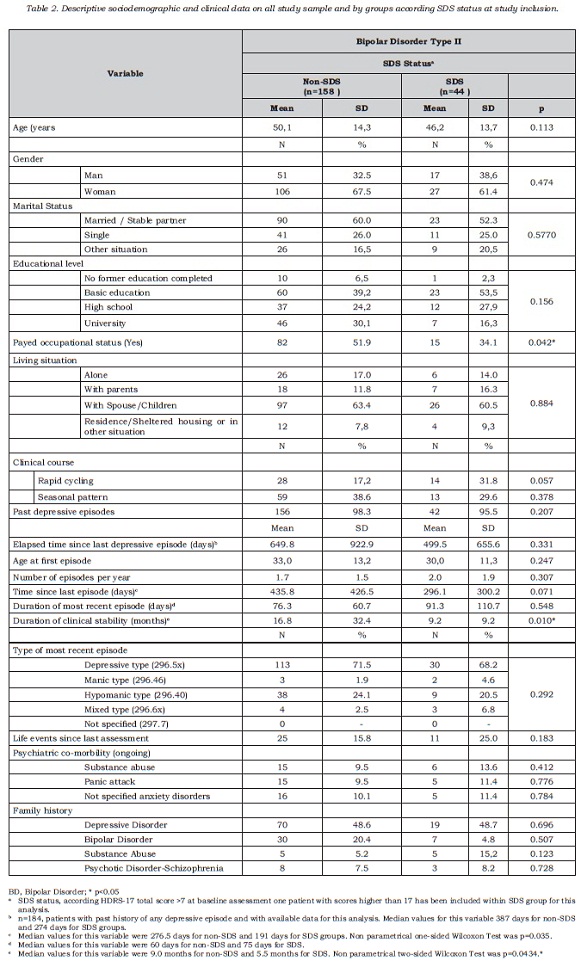
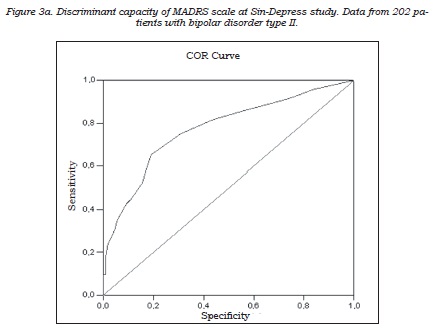
The correlation between the self-applied test and the clinical depression rating scales showed that MADRS score, but not the HDRS17 score, best correlated with the self-applied test. The correlation coefficients with CES-D were 0.59 (p<0.0001) for MADRS (figure 2) and 0.52 for HDRS-17 (p<0.0001). Both instruments were administered during the same interview and they were highly correlated (r = 0.80, p<0,0001); nevertheless, the partial correlation analysis identified the MADRS scale as the instrument most congruent with the self-applied questionnaire: partial coefficients with CES-D were r = 0.33, p<0.0001 for MADRS and r = 0.10, p<0.172 for HDRS-17, respectively. The discriminant capacity of the MADRS scale to identify the patients positive for depression on the self-applied test (“gold standard”) was subsequently analyzed, using the diagnostic performance or ROC curve (figure 3). The area under the curve obtained was close to 1, indicating a good discriminant capacity for MADRS to identify those patients reporting a high frequency of depressive symptoms in the period close to the visit.
Change in SDS status, evolution of depressive symptoms and incidence of SDS during follow-up
The prospective 16-week study consisted in a sample of 181 type II BD patients (89.6% of the baseline sample) who underwent follow-up assessment within the established time period. Results of the sample of 202 patients that completed both visits regardless time window for final visit are also reported regarding their clinical measurements (table 4).
The MADRS score corresponding to the baseline cut-off point defined in the HDRS-17 scale was 8.5, obtained by regression analysis as it has been described elsewhere (20). According to this cut-off point, over half of the patients with subclinical depression at baseline (55%, 23 out of 42) changed SDS status after 16 weeks, with depressive symptoms remitting to normal, whereas for the remaining patients this was not the case. Few of the patients in the SDS group suffered relapses during this period (3/42, 7%). Mean global reduction of symptoms in the SDS group at the end of follow-up was -2.3 points in the MADRS scale (95% IC = -4.1 to -0.5).
As for the incidence of SDS, 20.7% of the non-SDS cases at baseline (25 out of 121 non-SDS without relapses) increased their depressive component, thus meeting the established criteria for clinically significant desease, and 4.7% (6 out 127) reported new episodes during the follow up period. As a summary measure, 29% of type II BD patients with a status of non-SDS in the baseline assessment obtained scores corresponding to mild depression on the MADRS scale at the end of the study (37 out 127).
Patients who reported important life events prior to the assessment (n=36), showed higher intensity on depressive symptoms, mean score of 5.5, (SD 3.8) on HDRS-17 within this group versus a mean score of 4.1, (SD 3.1) for the group not reporting serious life events (n=166); the mean difference between both groups was 1.4 points on this scale (95% CI 0.2 to 2.6, p=0.021). An increase in depressive symptoms, in patients registering an important life events during follow-up (25 out of 196, 13%; with a mean increase of 5.5 points, SD 10 on the MADRS scale; 95% CI = 1.4 to 9.7) was found, at variance of those registering no such events (171 out 196, 87%) who showed little variation (mean (SD) -0.04 (4.9); 95% CI = -0.8 to 0.7). The mean difference observed between both groups was 5.6 points on MADRS (SD = 5.7; 95% CI = 3.1 to 8.0, p<0.0113).
Predictive value of depressive symptoms regarding recurrence
Considering the entire sample of type II BD patients, 11 patients presented new episodes of any polarity related to bipolar disorder during the study period (5.5%). A positive association between subclinical depressive symptoms and a greater risk of suffering a new episode was investigated. The analysis first performed considered the definition of SDS status as per the HDRS-17 score, followed by a further analysis of the total-SDS status (according to HDRS-17 and/or CES-D). No risk of recurrence was associated with any of the different definitions of SDS status. No significant differences were found between groups when analyzing recurrences in either the SDS group (χ2 = 0.246, d.f = 1, p = 0.7), or in the total-SDS group (χ2 = 0.149, g.l = 1, p = 0.365). In this last case, the risk of suffering a new episode in the 16-week period was 3.9% in the total-SDS group (4 out of 103) versus 7.1% (7 out of 98) in the non-total-SDS group.
Subclinical depression and social-occupational impact
Social-occupational scores registered in type II BD patients showed a slight decline that remained at the follow-up visit (table 4). Greater impairment in baseline functional status was found in the SDS group, involving more difficulties related to social, occupational or school life. In addition, there was a smaller proportion of patients in paid employment (table 2) within the SDS group.
With regards to social adjustment, the SDS group patients also showed a worse baseline adjustment than for non-SDS patients (p=0.007), although no significant difference between groups was found at the final evaluation (p=0.051). Analyzing differences regarding factors described by the SASS scale, it was found that both patient groups differed at their baseline assessment in all SASS scale factors, except those related to social and intellectual interests for which patients obtained similar results regardless of their baseline SDS status. At the end of the follow-up period, however, both groups only differed in the factor related to family relationships and behavioral strategies (p=0.02), the rest of results being comparable.
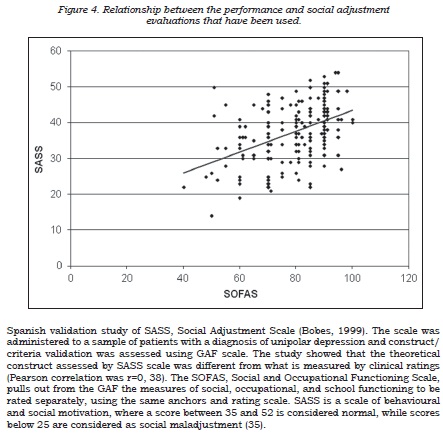
An inverse correlation between the depression rating and social-occupational performance scales was observed (table 5), this suggesting that depressive symptoms have a negative impact on the patient’s social-occupational performance and social adjustment, although this only explains part of the observed variability. In this context, it was also found that, although both scales were significantly correlated, they evaluate different aspects of patients’ lives (figure 4). A good correlation was found between the self-applied depression questionnaire and the SASS factor regarding to patient’s functioning on the job and leisure.
Discussion
The results of the SIN-DEPRES-Type II BD study are consistent with prior studies and confirm the presence of an important depressive component in patients with clinically stable type II BD. Although these patients receive maintenance treatment, a significant number of them continue to have mild or subclinical symptoms of depression which go unnoticed. They represented 21.3% of the patients as evaluated by clinical interview, and even it went up to close to 51% when the evaluation was completed with specific self-applied tools.
The observed SDS rates were similar in the type I and type II BD subsamples, without significant differences in prevalence rates, i.e. SDS prevalence of 15.9% (CI = 12.8 to 19.0%) for type I BD (20) being observed. In prior studies, the two disorders showed similar features, i.e. largely characterized by moderate or subsyndromal depressive syndromes during the course of the condition (7-9), and also similar periods of time that patients present with these symptoms (16). Therefore, type II BD is not the mildest form of bipolar disorder; it is also a serious condition.
The socio-demographic and clinical differences between BD I and II patients, suggest that the sample is representative of the BD II clinical population. This is based on the fact that differences observed between type I and type II patients are consistent with those commonly described and expected (19). Among type II BD, there was a higher proportion of women and a higher proportion of patients with an independent living style. Regarding clinical aspects, type II BD patients were older at the age of first episode, on average suffered more episodes per year, and the past history of depressive episodes was more common than in type I BD. Therefore, our results, related to type II BD patients could be considered as holding an appropriate external validity.
The characterization of type II BD patients suffering SDS at the baseline visit suggests that depressive components identified during the clinical interview could be, in some patients, related to the recovery from a previous episode as it was detected in our study: In other words, depressive symptoms would be more severe in patients who suffered an episode shortly before the control visit. The performed analysis, however, should be taken with caution due to the dissimilar sizes of the compared groups and the multiple comparisons performed. Our results should therefore be confirmed by future studies with comparable samples in relation to group size and SDS status.
Furthermore, SDS patients were clinically characterized by greater intensity of all the symptoms evaluated by the HDRS-17 scale, and even more severity regarding symptoms like insomnia, psychic anxiety and interference of these symptoms on patient’s job or main activity. These findings suggest the relevance of the impact of depression symptoms on patients’ lives. At the end of follow up period, at least half of the patients who presented SDS at the baseline assessment had reduced their depressive symptoms to levels that are considered to be normal. Factors related to this normalization have not been investigated and the end of the follow-up program between study visits was variable, depending on the regular practice at each centre.
Depressive symptoms evaluated by the HDRS-17 scale were specific for type II BD patients; this group of patients showed more intense depressive symptoms when compared with the results obtained in the CS group. The estimated prevalence of SDS was valid and specific for the studied population and differs from what would have been found in non-clinical samples i.e. from the general population. The interpretation of these results should consider the differences observed in socio-demographic variables between the group of patients and the healthy volunteers. Hence, the CS group was younger than the group of patients with type II BD. The impact of this variable on the prevalence of depression has been established in epidemiological studies (44), showing that younger people are exposed to a greater risk of mental disorders (such as mood, anxiety and alcohol disorders). Although a psychiatric diagnosis was cause for exclusion from the CS group, we believe that the effect of this variable on the detection of depressive symptoms was limited.
Although there is no consensus in the definition of SDS used in the literature, the results of this study are in line with published prevalence estimations for subsyndromal depression. Bennazzi et al (4) found residual depressive symptoms in 44.9% of the evaluated patients with type II BD, and the Stanley Foundation Bipolar Network study found a prevalence of 38%, using the Inventory of Depressive Symptomatology Clinicians Rating (ICS-C) (15). Comparisons are difficult because of the heterogeneous definitions used in the different studies (45).
The first prevalence rate derived from the HDRS-17 scale provides a figure based on a robust clinical tool widely used in psychiatry. On the other hand, the information about emotional status provided by the patient by self-assessment seems to adequately complement the information obtained from the interview, as mentioned by some authors (39). It is important to mention that specifically for type II BD, a poorer awareness of their illness than patients with bipolar I (46) has been described and, moreover, variations on this factor can contribute to the difficulty of clinical assessment during follow up visits. In this way, the relationship between the level of insight regarding depression status and self-reporting measures was also analyzed for type II BD patients. Correlation results showed no significant relationships between level of insight (i.e. regarding depression as measured by item 16 on HDRS-17) and CES-D total score (r= -0.08, p= 0.28) neither with SASS total score (r= -0.07, p= 0.28). This means that, at least on clinicaly stable type II BD patients, self-reported information about depressive mood and social adjustment seems not be influenced by the current patient’s level of insight.
This study shows that patients suffering from depressive symptoms close to a routine visit that only obtained high scores in the self-applied questionnaire, not only exhibited less prominent depressive symptoms, thus obtaining normal results in a clinical interview, but were also better adjusted to their social environment and presented better social and occupational performance compared with patients that were detected by both methods. In relation to the associated factors, older patients presented a greater variability in the duration of clinical stability, with a mean difference of nearly one year in maintaining clinical stability, than those patients detected by both methods.
These findings allow to hypothesize, on the one hand, that self-referred depressive symptoms can reflect the impact of the chronicity of the disorder on the patient’s emotional status and, on the other hand, that, as they record the most cognitive aspects involved in depression, they could represent the emotional status prior to the development of more intense symptoms, thus preceding clinically manifest depressive symptoms. This would mean that self-referred symptoms could, among other, represent a possible predictive factor for subsequent relapses throughout the clinical course of the disease, however this relationship needs to be addressed in future studies. These findings open lines of research about the factors related to the natural course of type II BD.
The CES-D scale was selected in our study because it best evaluates the cognitive features of depression, whereas previous studies used Life Chart Methods (LCM). That mainly evaluates subjective mood. The CESD scale represents a reliable alternative to LCM, since it is a detailed measurement of mood at a time close to the visit, not requiring significant memory effort by the patient and, therefore, being an alternative for complementing mood evaluation. The CES-D is therefore a tool to be considered for evaluating type II BD patients during follow-up visits. This scale was designed to be used in epidemiological studies in the general population, although it has also been clinically validated in Spain in patients diagnosed with, and treated for, affective disorders (32).
As for the detection of subclinical depressive symptoms, the results of this study support the use of the MADRS scale in type II BD before the widely used HDRS-17 scale. The MADRS scale provides results that were not only more consistent with the self-reported measures, but also were better related to the patient’s social-occupational performance and social adjustment in his/her daily life. Accordingly, we suggest the convenience of using the MADRS scale in the follow-up assessments on type II BD patients, and as well as proposing a cut-off point of 5, instead of either 7 or 8, to detect patients with subclinical symptoms.
In relation to the incidence of SDS among type II BD patients in a 16-week follow-up period (i.e. medium term), around 20% of the type II BD patients, who were free from symptoms at baseline, suffered significant increases of depressive symptoms, and up to one third of the sample developed mild or subclinical symptoms at the end of the study period (MADRS scores over cut-off values for mild depression). In addition, an increase in the score of around 5 points was significantly associated with the occurrence of important life events.
Subclinical symptoms and social-occupational performance
The relationship between depressive symptoms and social-occupational performance, showing an expected inverse relationship, was confirmed in our study. The greater the depressive component, the worse the performance. Subsyndromal depressive symptoms evaluated by the HDRS-17 scale seem to be associated with worse psychosocial performance compared with asymptomatic patients (15,17,47). As such, fluctuations in the depressive components result in variations of the socio-occupational functioning and social adjustment.
The SASS scale was specifically developed to evaluate the efficacy of new anti-depressive treatments, and it was validated in the general population and in patients with unipolar depression. Clinical trials indicate that the information provided by the scale during remission is independent from the evaluation performed with depressive symptom intensity scales (43). This construct independence, for instance from the HDRS scale, was confirmed in the Spanish validation study; it has also been found that this scale discriminates between patients with different degrees of depression (34). Consistent with this finding, the SDS patient group in the SIN-DEPRES-type II BD study obtained lower scores than the non-SDS patients, not reaching social maladjustment. We believe that the scale is sensitive to the change of status in a patient, reflects his/her disease perception and is therefore a useful tool for evaluating the response to treatment in this context.
The analysis of the course of social adjustment during the follow-up period showed that the differences found between groups according to the presence of SDS tended to disappear at the end of the study. When performing the same analysis considering the total-SDS group variable (that is, including SDS detection by any method), the baseline differences remained at the end of the study. The total SDS group obtained a mean SASS score of 34.8 (SD 8.2, 95% CI 33.2 to 36.5), less than the non-SDS group scoring a mean of 40.6 (SD 6.6. 95% CI 39.2 to 41.9) (p<0.0014), and showing poorer social adjustment. These differences remained at the final assessment (p=0.0269) both with regards to total score and to all the SASS factors analyzed. Once again, this result suggests that the information obtained from self-applied tests acceptably complements the information derived from the clinical interview; so, a combination of the two assessments provides results concerning these symptoms’ impact on social-occupationalperformance which allows to differentiate both groups (when defined using both tools).
Predicting recurrence
Different studies have shown that SDS in BD are associated with an increased risk of recurrence (15). In type II BD, the association between depressive symptoms and the risk of suffering new episodes in our study was not positive.
A considerably low rate of recurrence was found in the present study compared to other prospective studies, which document a 24.3% recurrence after 6 months (14), or 35.9% after one year in type I BD (48) and 61.2% after two years (49). The NIMH Collaborative Study of Depression, with 152 cases of type I BD, found recurrence rates of 48% and 57% after 1 year (50). The low recurrence rate in the Sin-Depres study could be explained in addition to a short follow-up period, by some other factors which could have contributed to our negative result, like the easy access to health care by the Spanish public health care system and the fact that all the patients were treated and prospectively followed up. A study with a longer follow-up period would provide a more precise determination or rule out the relationship between SDS and risk of recurrence in ambulatory patients. In the STEP-BD study, the mean time to recurrence of any mood episode was 44.9 weeks (95% CI = 37.6 to 53.1) (14). This information, which had not been published when the present study was planned, should therefore guide the design of future research.
The present study has some limitations. Only ambulatory patients were included and since no inpatient population was studied, this group may not be representative of a non-epidemiologically selected sample of patients with BD. The selection of our cohort probably introduced some bias in the results, as it comprises a large number of patients with good adherence to follow-up programs, a better one than it is usually found in clinical practice; this could partly explain the finding of a low recurrence rate. Another limitation is that, in the definition of adopted prevalence, the time that symptoms have been present is not considered. In order to achieve an appropriate predictive power, we believe that, in future studies, clinical evaluations should not only contemplate depressive symptoms but also be supplemented with evaluations of manic-hypomanic polarity symptoms.
In conclusion, type II BD is a chronic affective disorder largely dominated by minor or subclinical symptoms of depression (according to the definition) as 7.3. in type I BD. Despite possible limitations of this study, the data highlights the frequency with which subclinical symptoms persist and wax and wane in ambulatory patients in whom the disorder is classified as clinically stable.
Acknowledgements
The authors thank the following organizations and people for their scientific and logistic contributions to the project; M. Puig and B. Gancedo at PSYNCRO, Neuropsychological Research Organization, S.L., Barcelona, Spain; M. De Gracia, Psychology Department, Basic Psychology Area, Universitat de Girona, Girona, Spain; J. Lahuerta, Medical Department, GlaxoSmithKline, S.A. The authors would like to acknowledge the following investigators taking part in the SIN-DEPRES Study Group (see appendix 1 for the investigators list) This study was funded by GlaxoSmithKline, S.A. Tres Cantos (Madrid), Spain.
References
1. American Psychiatric Association. Practice guideline for the treatment of patients with bipolar disorder. American Psychiatric Association. Am J Psychiatry. 1994;151(Suppl):1-36. [ Links ]
2. American Psychiatric Association. Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry. 2002;159(4 Suppl):1-50. [ Links ]
3. Angst J. Long term treatment of depression. Chichester: Wiley; 1992. [ Links ]
4. Benazzi F. Prevalence and clinical correlates of residual depressive symptoms in bipolar II disorder. Psychother Psychosom. 2001;70:232-8. [ Links ]
5. Benazzi F. Residual depressive symptoms in bipolar depression. Am J Psychiatry 2002 May;159(5):882. [ Links ]
6. Post RM, Denicoff KD, Leverich GS, et al. Morbidity in 258 bipolar outpatients followed for 1 year with daily prospective ratings on the NIMH life chart method. J Clin Psychiatry. 2003;64:680-90. [ Links ]
7. Judd LL, Schettler PJ, Akiskal HS, et al. Long-term symptomatic status of bipolar I vs. bipolar II disorders. Int J Neuropsychopharmacol. 2003;6:127-37. [ Links ]
8. Judd LL, Akiskal HS. Depressive episodes and symptoms dominate the longitudinal course of bipolar disorder. Curr Psychiatry Rep. 2003;5:417-8. [ Links ]
9. Judd LL, Akiskal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60:261-9. [ Links ]
10. Post RM, Leverich GS, Altshuler LL, et al. An overview of recent findings of the Stanley Foundation Bipolar Network (Part I). Bipolar Disord. 2003;5:310-9. [ Links ]
11. Paykel ES, Abbott R, Morriss R, et al. Sub-syndromal and syndromal symptoms in the longitudinal course of bipolar disorder. Br J Psychiatry. 2006;189:118-23. [ Links ]
12. Fava GA. Subclinical symptoms in mood disorders: pathophysiological and therapeutic implications. Psychol Med. 1999;29:47-61. [ Links ]
13. Judd LL, Schettler PJ, Akiskal HS, et al. Residual symptom recovery from major affective episodes in bipolar disorders and rapid episode relapse/recurrence. Arch Gen Psychiatry. 2008;65:386-94. [ Links ]
14. Perlis RH, Ostacher MJ, Patel JK, et al. Predictors of recurrence in bipolar disorder: primary outcomes from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEPBD). Am J Psychiatry. 2006;163:217-24. [ Links ]
15. Altshuler LL, Post RM, Black DO, et al. Subsyndromal depressive symptoms are associated with functional impairment in patients with bipolar disorder: results of a large, multisite study. J Clin Psychiatry. 2006;67:1551-60. [ Links ]
16. Joffe RT, MacQueen GM, Marriott M, et al. A prospective, longitudinal study of percentage of time spent ill in patients with bipolar I or bipolar II disorders. Bipolar Disord. 2004;6:62-6. [ Links ]
17. MacQueen GM, Marriott M, Begin H, et al. Subsyndromal symptoms assessed in longitudinal, prospective follow-up of a cohort of patients with bipolar disorder. Bipolar Disord. 2003;5:349-55. [ Links ]
18. Vieta E, Gasto C, Otero A, et al. Differential features between bipolar I and bipolar II disorder. Compr Psychiatry. 1997;38:98-101. [ Links ]
19. Vieta E, Suppes T. Bipolar II disorder: arguments for and against a distinct diagnostic entity. Bipolar Disord. 2008;10:163-78. [ Links ]
20. Vieta E, Arce R, Jiménez-Arriero MA, et al. Detection of subsyndromal depression in bipolar disorder: cross-sectional and 4-month prospective follow up study at community mental health services (SIN-DEPRES). J Clin Psychiatry. 2010;71:1465-74. [ Links ]
21. American Psychiatric Association. DSM-IV-TR. Manual de diagnóstico y estadístico de los trastornos mentales, 4ta edición, texto revisado. Barcelona: Editorial Masson; 2002. [ Links ]
22. American Psychiatric Association. Diagnostic and statistical manual for mental disorders. DSM-IV-TR. 4th ed. Revised Text. Washington: APA; 2000. [ Links ]
23. Vieta PE, Torrent FC, Martinez-Aran A, et al. [A user-friendly scale for the short and long term outcome of bipolar disorder: the CGI-BP-M]. Actas Esp Psiquiatr. 2002;30:301-4. [ Links ]
24. Spearing MK, Post RM, Leverich GS, et al. Modification of the clinical global impressions (CGI) scale for use in bipolar illness (BP): the CGI-BP. Psychiatry Res. 1997;73:159-71. [ Links ]
25. Tormo Díaz M, Dal-Ré R, Pérez Albarracín G. Ética e investigación epidemiológica: principios, aplicaciones y casos prácticos. Recomendaciones de la Sociedad Española de Epidemiología (SEE) sobre la revisión de los aspectos éticos de la investigación epidemiológica. Murcia: SEE; 1998. [ Links ]
26. Dal-Re R, Tormo MJ, Perez G, et al. [Ethical review of epidemiologic studies: a need and a proposal]. Med Clin (Barc). 1998;111:587-91. [ Links ]
27. Maier W, Philipp M, Heuser I, et al. Improving depression severity assessment--I. Reliability, internal validity and sensitivity to change of three observer depression scales. J Psychiatr Res. 1988;22:3-12. [ Links ]
28. Bobes J, Bulbena A, Luque A, et al. [A comparative psychometric study of the Spanish versions with 6, 17, and 21 items of the Hamilton Depression Rating Scale]. Med Clin (Barc). 2003;120:693-700. [ Links ]
29. Lobo A, Chamorro L, Luque A, et al. [Validation of the Spanish versions of the Montgomery-Asberg depression and Hamilton anxiety rating scales]. Med Clin (Barc). 2002;118:493-9. [ Links ]
30. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382-9. [ Links ]
31. Weissman MM, Sholomskas D, Pottenger M, et al. Assessing depressive symptoms in five psychiatric populations: a validation study. Am J Epidemiol. 1977;106:203-14. [ Links ]
32. Soler J, Perez-Sola V, Puigdemont D, et al. Estudio de validación del Center for Epidemiologic Studies-Depresion (CES-D) en una población española de pacientes con trastornos afectivos. Actas Luso Esp Neurol Psiquiatr Cienc Afines. 1997;25:243-9. [ Links ]
33. Radloff JS. The CES-D scale: a self-report depression scale for research in the general population. APM. 1977;1:385-401. [ Links ]
34. Bobes J, Gonzalez MP, Bascaran MT, et al. [Validation of the Spanish version of the social adaptation scale in depressive patients]. Actas Esp Psiquiatr. 1999;27:71-80. [ Links ]
35. Bosc M, Dubini A, Polin V. Development and validation of a social functioning scale, the social adaptation self-evaluation scale. Eur Neuropsychopharmacol. 1997;7(Suppl 1):S57-70. [ Links ]
36. Goldman HH, Skodol AE, Lave TR. Revising axis V for DSM-IV: a review of measures of social functioning. Am J Psychiatry. 1992;149:1148-56. [ Links ]
37. Weissman MM, Olfson M, Gameroff MJ, et al. A comparison of three scales for assessing social functioning in primary care. Am J Psychiatry. 2001;158:460-6. [ Links ]
38. Vieta E, Sanchez-Moreno J, Lahuerta J, et al. Subsyndromal depressive symptoms in patients with bipolar and unipolar disorder during clinical remission. J Affect Disord. 2008;107:169-74. [ Links ]
39. Snaith RP, Harrop FM, Newby DA, et al. Grade scores of the Montgomery-Asberg depression and the clinical anxiety scales. Br J Psychiatry. 1986;148:599-601. [ Links ]
40. Altman DG. Practical statistics for medical research. London: Chapman and Hall; 1991. [ Links ]
41. Senn S. Statistical issues in drug development. Statistics in practice. 1st ed. Chichester (UK): John Willey; 1997. [ Links ]
42. Landis JR, Kich G. The measurement of measurer agreement for categorical data. Biometrics. 1977;33:159-74. [ Links ]
43. Bosc M. Assessment of social functioning in depression. Compr Psychiatry. 2000;41:63-9. [ Links ]
44. Alonso J, Angermeyer MC, Bernert S, et al. Prevalence of mental disorders in Europe: results from the european study of the epidemiology of mental disorders (ESEMeD) project. Acta Psychiatr Scand Suppl. 2004;21-7. [ Links ]
45. Vieta E, Sanchez-Moreno J, Bulbena A, et al. Cross validation with the mood disorder questionnaire (MDQ) of an instrument for the detection of hypomania in Spanish: the 32 item hypomania symptom check list (HCL-32). J Affect Disord. 2007;101:43-55. [ Links ]
46. Pallanti S, Quercioli L, Pazzagli A, et al. Awareness of illness and subjective experience of cognitive complaints in patients with bipolar I and bipolar II disorder. Am J Psychiatry. 1999;156:1094-6. [ Links ]
47. Judd LL, Akiskal HS, Schettler PJ, et al. Psychosocial disability in the course of bipolar I and II disorders: a prospective, comparative, longitudinal study. Arch Gen Psychiatry. 2005;62:1322-30. [ Links ]
48. Bromet EJ, Finch SJ, Carlson GA, et al. Time to remission and relapse after the first hospital admission in severe bipolar disorder 6. Soc Psychiatry Psychiatr Epidemiol. 2005;40:106-13. [ Links ]
49. Gitlin MJ, Swendsen J, Heller TL, et al. Relapse and impairment in bipolar disorder. Am J Psychiatry. 1995;152:1635-40. [ Links ]
50. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59:530-7. [ Links ]