Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Psiquiatría
Print version ISSN 0034-7450
rev.colomb.psiquiatr. vol.40 suppl.1 Bogotá Oct. 2011
Artículos originales
What is the Contribution of Executive Dysfunction to the Cognitive Profile of Bipolar Disorder? A Well-Controlled Direct Comparison Study
¿Cuál es la contribución de la disfunción ejecutiva al perfil cognitivo del trastorno bipolar? Un estudio comparativo bien controlado
Juan Lopera-Vasquez1
Vaughan Bell1
Carlos López-Jaramillo2
1Research Group on Psychiatric Disorders, Department of Psychiatry, School of Medicine, University of Antioquia, Medellín, Colombia. Chairman Department of Psychiatry School of Medicine, University of Antioquia, Medellín, Colombia.
2MD Psychiatrist. Coordinator of the Psychiatry Research Group (GIPSI) and Chair of the Department of Psychiatry at the Medicine School of the University of Antioquia. Medellín, Colombia.
Conflicts of interest: The authors have not conflicts of interest.
Corresponding author
Carlos López-Jaramillo
Departamento de Psiquiatría,
Facultad de Medicina Universidad de Antioquia
Calle 64 No. 51 D 38 Medellín, Colombia.
clopez@medicina.udea.edu.co
Recibido para evaluación: 17 de junio del 2011 Aceptado para publicación: 30 de junio del 2011
Abstract
Background: Large scale neuropsychological studies of patients with bipolar disorder have reported verbal memory and executive function deficits that persist during remission. A recent analysis by Thompson et al. (2009) indicated that verbal memory deficits could be entirely explained by the statistical variance attributed to primary executive function deficits. This study tests the hypothesis that verbal memory deficits in bipolar patients are largely the result of executive difficulties by direct comparison of verbal neuropsychological tests primarily differing in their executive load as well as examining potential interactions with medication status. Methods: 33 Bipolar I patients not taking medication, 40 Bipolar I patients taking medication, and 28 healthy controls were compared on measures of IQ, verbal fluency, category fluency, verbal recall, and category prompted recall. Results: After controlling for IQ, performance on tasks that involved additional executive involvement was significantly worse. Medication had a small but reliable effect on cognitive performance. Conclusions: The results provide support to the hypothesis that the most significant source of cognitive impairment in bipolar disorder stems from executive impairment and that verbal memory deficits may arise as a result of this, rather than from primary impairment to core verbal memory mechanisms.
Key words: Bipolar disorders, memory, neuropsychology, executive function.
Resumen
Introducción: Los estudios neuropsicológicos a gran escala de pacientes con trastorno bipolar han reportado déficits en la memoria verbal y en la función ejecutiva que persisten durante la remisión. Un análisis reciente realizado por Thompson et al. indicó que los déficits en la memoria verbal podían explicarse enteramente por la varianza estadística atribuida a los déficits en la función ejecutiva primaria. Este estudio demuestra la hipótesis de que los déficits en la memoria verbal de pacientes bipolares son mayormente el resultado de las dificultades ejecutivas mediante una comparación directa de pruebas neuropsicológicas verbales, que difieren principalmente en su carga ejecutiva, como también mediante la examinación las interacciones potenciales con el estado de la medicación. Métodos: Se compararon 33 pacientes con trastorno bipolar I que no estaban tomando medicamentos con 28 controles saludables respecto a sus medidas de coeficiente intelectual (CI), fluidez verbal, fluidez de categorías, memoria verbal y memoria alentada por categorías. Resultados: Después de realizar controles relacionados con el CI, el desempeño que requería un mayor involucramiento ejecutivo era significativamente peor. Los medicamentos tenían un efecto pequeño pero confiable sobre el desempeño cognitivo. Conclusiones: Los resultados soportan la hipótesis de que la fuente más significativa de trastornos cognitivos en el trastorno bipolar es el trastorno ejecutivo y que pueden surgir déficits en la memoria verbal como resultado de este, y no de un trastorno primario de los mecanismos centrales de la memoria verbal.
Palabras clave: Trastorno bipolar, memoria, neuropsicología, función ejecutiva.
Introduction
Meta-analysis of neuropsychological studies have reported neurocognitive deficits in patients with bipolar disorder that persist when euthymic and during periods of remission (Robinson et al., 2006; Torres et al., 2007; Bora et al., 2009; Kurtz and Gerraty, 2009) with verbal memory and executive function being the most commonly encountered areas of cognitive impairment. The fact that these deficits are present when patients are asymptomatic and, albeit less consistently, in non-affected family members (Balanzá-Martínez et al., 2008) indicates that reduced cognitive performance in key areas could be a vulnerability marker or endophenotype for the disorder (Jamrozinski, 2010).
Nevertheless, the issue of the neuropsychological impairments in bipolar disorder goes beyond the basic science and has clear clinical implications as cognitive difficulties have been reported to be one of strongest predictors of functional impairment in bipolar patients (on a par with mood symptoms; Sanchez-Moreno et al., 2009a) and remain a strong and significant predictor of functional outcome even when mood symptoms, hospitalisations, suicide attempts and demographic variables are controlled for (Wingo et al., 2009a). Similarly, a study by Gualtieri et al. (2008) found that bipolar has the highest rate (30%) of severe cognitive impairment among the mood disorders, defined as cognitive performance two standard deviations below the population mean in at least two cognitive domains.
But despite the fact that cognitive difficulties are clearly associated with bipolar disorder, the core neurocognitive impairments identified as possible risk factors for the condition have recently been questioned. Although verbal memory and executive functions have been associated with the condition, a recent analysis by Thompson et al. (2009) has suggested that the verbal memory difficulties could be entirely explained by the executive difficulties rather than representing a distinct domain of cognitive impairment. Furthermore, recent meta-analyses of structural imaging studies have been provided no evidence of changes in areas of the temporal cortices that would be typically associated with primary verbal memory deficits (Arnone et al., 2009; Vita et al., 2009) while reductions in the volume of pre-frontal cortex, more associated with executive function, have been associated with bipolar disorder (Arnone et al., 2009).
One of the obstacles to clearly characterising the cognitive impact of bipolar is the significant variability seen across studies in terms of patient selection. In a recent review of neurocognitive findings, Jamrozinski (2010) notes that “This heterogeneity in results has been a challenge to reviews and meta-analytic studies, and the discrepancies cannot be resolved as long as data on confounding variables are incompletely reported in original studies” and highlights how differences in diagnoses, active symptoms, medication status, history of substance abuse, patient demographics and neuropsychological assessment methods contribute towards ambiguous results.
Nevertheless, better controlled studies are starting to appear that include euthymic patients without significant differences in active symptoms that examine the impact of substance misuse (Sanchez-Moreno et al., 2009b) or evaluate the impact of psychiatric medication (Goswani et al., 2009). One of the most cleanly controlled studies in this regard is from Lopéz-Jaramillo et al. (2010a, 2010b) which includes groups of euthymic bipolar patients both with and without medication, who additionally do not differ on the clinical and demographic variables that have been identified as possible confounding variables.
Additionally, these participants completed an extensive neuropsychological battery that allows a direct comparison between verbal memory tasks that are known to differ primarily in their reliance on the executive system owing to the fact that category prompted recall is known to require less spontaneous use of executive function-based organisational recall strategies (Shimamura, 1995). These evaluations include letter and category fluency, with the frontal-executive having been shown to be much more strongly involved in letter fluency than category fluency (Baldo et al., 2006; Birn et al., 2010); and free recall and category prompted recall, which similarly have been shown to differ with free recall showing much stronger frontal-executive involvement than category cued recall (Wheeler et al., 1995; Taconnat et al., 1995).
This allows a test of Thompson et al.’s (2009) suggestion that verbal memory deficits in bipolar are largely accounted for by executive difficulties - although while Thompson et al. examined the hypothesis by statistically controlling for variance among tests, the current data set allows this hypothesis to be tested by directly comparing performance on tests that differ primarily in their executive involvement. On this basis, we hypothesise that bipolar patients will show greater impairment in letter fluency than category fluency, and free recall compared to category cued recall, indicating a relatively greater impairment in executive function.
Method
Participants
We evaluated a sample of 101 participants for the study comprising 33 patients diagnosed with Bipolar Disorder I not taking medication, 40 patients diagnosed with Bipolar Disorder I that were prescribed medication, and 28 healthy controls.Those patients who did not take medication did so through their own choice des-pite recommendations to the contrary by their doctor and understanding the risks of not doing so. This group agreed to have regular monitoring of their symptoms and this was noted in the consent form they signed. All participants were evaluated by a psychiatrist who performed the Diagnostic Interview for Genetic Studies (DIGS), which has been confirmed as a valid and reliable diagnostic measure in a Spanish-speaking population (Palacio et al., 2004; Roca et al., 2007). This evaluation was used to confirm that patients fulfilled the DSM-IV criteria for this disorder. The research protocol used was approved by the Ethics Committee, and all participants read, understood, and signed an informed consent form before being assessed.
Patients
All BD-I patients from a local mood disorders clinic (University of Antioquia Mood Disorder Clinic, University Hospital San Vicente de Paúl, Medellín, Colombia) and who fulfilled the inclusion criteria were invited to participate in the study. All patients reported being euthymic for at least six months, confirmed by clinical record review, and the presence of residual affective symptoms was controlled for, as they needed to score < 8 on the Zung Self-Rated Depression Scale (ZSDS) and < 6 on the Young Mania Rating Scale (YMRS). Furthermore, patients were excluded if individuals had consumed illicit substances or benzodiazepines during the four weeks prior to the assessment, or had other psychiatric or neurological disorders, such as epilepsy, mental retardation (IQ < 70), or any treatment with electroconvulsive therapy. Exclusion criteria were confirmed by a review of the case notes. All participants had to be between 18 and 60 years of age and have had 5 to 16 years of schooling, restricting the potential influence of low illiteracy levels on neuropsychological performance. Recruited patients were divided into two groups, depending on whether they were currently prescribed medication or not. Out of the patient group prescribed medication, 20 were prescribed lithium, 11 were prescribed valproate, one was additionally prescribed an antipsychotic and one was additionally prescribed an antidepressant at time of testing.
Controls
Healthy controls conformed to the same exclusion criteria used for the patient participants and who were also assessed with the DIGS to confirm the absence of psychopathology.
Materials
Neuropsychological Assessment
All participants completed an extensive neuropsychological battery which is fully described in López-Jamarillo et al. (2010b). With regards to this particular study, results from five neuropsychological tests are included in the analysis. All participants were assessed with the Spanish version of the Wechsler Adult Intelligent Scale 3rd edition.
Phonological verbal fluency was assessed by the use of the standard version of this assessment where participants are asked to name as many words as they can think of in one minute. Three trials of the test were run, using the prompt letters ‘F’, ‘A’ and ‘S’. Categorical verbal fluency was assessed by the standard version of this test where participants are asked to name as many examples of each category as possible, using the prompts ‘animals’ and ‘fruits’. As discussed in the Introduction, performance -measured by correct responses- on these phonological and categorical verbal fluency has been shown to differentially involve the prefrontal and temporal cortices (e.g. Baldo et al., 2009; Birn et al., 2010).
Free recall was assessed by the use of first free recall condition of the Spanish version of the California Verbal Learning Test (CVLT; Jacobs et al., 1997) where participants are asked to recall as many of the words as they can in any order they wish from a list of 16 read out by the assessor. Categorical recall was assessed by the use of the first category prompted recall trials of the semantic memory with associative increment test (SMAI; Pineda and Ardila, 1991) where participants are given four category headings to assist with recall of the items.
Analysis
Potential demographic differences between groups were assessed with one-way between groups analysis of variance (ANOVA), for continuous variables shared between all groups, and t-tests for continuous clinical variables only relevant to patients. Non-parametric Kruskal-Wallis tests were used to compare the three groups on non-continuous demographic variables (such as marital and occupational status). A one-way between groups multivariate analysis of covariance (MANCOVA) was completed to examine differences in neuropsychological test performance between healthy controls, patients without medication and patients with medication. Four dependent variables were included - number of correct items on: phonological verbal fluency, categorical verbal fluency, CVLT free recall, semantic memory with associative increment test category recall. WAIS IQ was included as a co-variable. Including all variables in a single analysis has the advantage of controlling for Type I errors by reducing problems with multiple comparisons.
Results
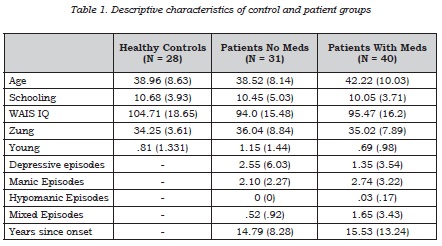
Means for demographic variables of the groups are displayed in Table 1. There were no significant differences between the three groups, compared using a one-way independent samples ANOVA, in terms of age (F(2, 95) = 1.743, p = .181), years of schooling =.182, p = .834), score on the (F(2, 95) Zung Self-Rating Depression Scale =.344, p = .710) or Young Mania Rating Scale (F(2, 93) = 1.206, p = .304) and no significant differences in LSD post-hoc comparisons between individual groups. However, when comparing WAIS IQ between groups there was a significant effect (F(2, 98) = 3.636, p = .03) with post-hoc LSD tests showing that while there was no difference between the patients group (p = .708) there was a significant difference between controls and unmedicated patients (p = .014) and controls and medicated patients (p = .027).
Using a non-parametric Kruskal-Wallis test to compare the three groups on non-continuous variables there was no significant difference between the three groups in terms of marital status (χ2 = .342, p = .806), occupational status (χ2 = .704, p = .806) and laterality (χ2 = 1.166, p = .558). There was a trend to significance in a comparison of the three groups in terms of previous abuse of drugs and alcohol (χ2 = 5.508, p = .08) although the trend no longer remains when the alpha is corrected for multiple comparisons.
When comparing the two patient groups using an independent samples t-test, there was no significant difference between number of depressive episodes (t(63) = .985, p = .328), number of manic episodes (t(63) = .915, p = .368), number of hypomanic episodes (t(56) = .838, p = .406) and years since first onset (t(65) = .274, p = .785). There was a trend to significance for number of mixed episodes (t(63) = 1.773, p = .081) but the trend no longer remains after correction for multiple comparisons.
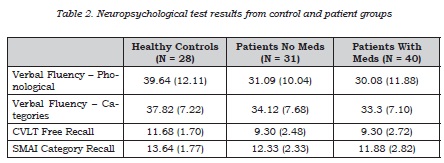
A one-way between groups MANCOVA was completed to examine differences in neuropsychological test performance between healthy controls, patients without medication and patients with medication. Four dependent variables were included â number of correct items on: phonological verbal fluency, categorical verbal fluency, CVLT free recall, semantic memory with associative increment test category recall with means displayed in Table 2. Owing to significant differences between patients groups and controls on Full Scale WAIS IQ, this was included as a co-variable in the analysis to control for the influence of differences in general cognitive functioning. Initial testing showed no violations of assumptions of normality, linearity, outliers, homogeneity of variance or multi-collinearity.
After adjusting for WAIS IQ, there was a significant effect of group on the combined variables (F(8, 188) = 2.458, Wilk's lambda = .82, p = .015). Considering the results of the dependent variables separately there was a significant effect on phonological verbal fluency (F(2, 97) = 3.207, p = .045) but no significant effect of categorical verbal fluency (F(2, 97) = .971, p = .382). Similarly there was a significant effect for CVLT free recall (F(2, 97) = 6.412, p = .002) but the effect for semantic memory with associative increment test category recall only just reached a trend for significance (F(2, 97) = 2.475, p = .089).
Post-hoc LSD tests were completed for the comparisons that showed overall significance (phonological verbal fluency and CVLT free recall). There was a significant difference in phonological verbal fluency between controls and patients with medication (p =.014) with the comparison between controls and patients without medication showing a trend to significance (p = .085) and no significant difference between patient groups (p = .465). For CVLT free recall there was a significant difference between both controls and patient with medication (p = .001) and patients without medication (p = .003) and no difference between patient groups (p = .873).
Discussion
The aim of the study was to examine whether neuropsychological tests of verbal memory that required a greater degree of executive control would be differentially impaired in patients with bipolar disorder. In line with the hypothesis, we found that performance on the more executively demanding tasks, phonological fluency and free recall, was significantly lower in patients than controls â in contrast to the less executively demanding tasks, category fluency and category cued recall, in which no differences between patients and controls were apparent. There was a slight trend for the patient group prescribed medication to perform worse than the unmedicated group, indicating a small but detectable impact of medication of cognitive performance, although this was far outweighed by the effect of bipolar disorder itself.
The pattern of results provides additional evidence that the most significant source of cognitive impairment in bipolar is from executive impairment and verbal memory deficits may arise as a result of this, rather than from primary impairment to core verbal memory mechanisms, in line with the results of Thompson et al. (2009). The pre-frontal cortex is known to be specialised for the use of active strategies for encoding and recall in episodic memory (Moscovitch, 1992) and it is notable that the tests used in the present study have been shown to preferentially engage this area (Baldo et al., 2006; Birn et al., 2010; Taconnat et al., 1995; Wheeler et al., 1995) providing additional evidence for the primacy of pre-frontal dysfunction in bipolar affective disorder.
Although individual structural neuroimaging studies have reported conflicting results, meta-analyses of structural imaging studies in bipolar have most typically reported lateral ventricular enlargement (Kempton et al., 2008; Arnone et al., 2009), increased rates of white matter hyperintensities (Kempton et al., 2008), grey matter reductions in the anterior cingulate and bilateral insula (Ellison-Wright and Bullmore, 2010) and an increased volume of the globus pallidus with reductions in whole brain and / or prefrontal structure volume (Arnone et al., 2009). It is notable that changes to the medial-temporal and hippocampal structures, that we would expect if verbal memory deficits results from impairment to primary memory mechanisms, are absent, while frontal changes that are more likely to impact on executive function are reliably reported.
Indeed, similar patterns of memory difficulty can be seen in patients with focal damage to prefrontal circuits, such as in degenerative disorder (Glosser et al., 2002) or acquired frontal lobe damage (Shimamura et al., 1991), and in schizophrenia where dysexecutive problems strongly contribute to episodic memory impairment (Ragland et al., 2009). However, it is important to note the considerable variability that has been reported between both patients and studies in this area with inconsistencies in medication regimes, clinical characteristics and demographics of the samples cited as contributory factors (e.g. Jamrozinski, 2010).
One particular issue has been the cognitive impact of medication which has received increasing attention in the literature with the results are largely in line with the findings from this study: medication has been found to have a detectable but minor effect of cognition (Goswami et al., 2009). Owing to its widespread use in bipolar disorder, lithium has been of particular interest (Fountoulakis et al., 2008) with a recent meta-analyses finding a small but reliable overall impact on cognition (Wingo et al., 2009b). With respect to other medications, benzodiazepines have a well established impact on memory (Vgontzas et al., 1995), preliminary evidence suggests that valproate and carbamazepine have been associated with lower cognitive function in comparison to patients treated with lamotrigine (Daban et al., 2006) while antipsychotics have been associated with reduced cognitive performance in bipolar patients (Dittmann et al., 2008; Jamrozinski et al., 2009; Arts et al., 2010) although it must be noted that the effect sizes are typically small and well-controlled studies are still lacking. Perhaps more pertinently, bipolar patients are commonly treated with a combination of medications and yet little is known about the cognitive impact of polypharmacy in this group (Fountoulakis, 2010).
However, there is a growing body of evidence that clinical characteristics, particularly the evolution of bipolar may lead to an increasing neurocognitive impact over-time. A meta-analysis specifically focused on structural brain changes during first episode bipolar patients reported a reduction in white matter volume that did not overlap with results reported from studies of chronic patients, indicating ongoing chan-ges associated with the evolution of bipolar disorder (Vita et al., 2009). A review of studies on the cognitive evolution of bipolar (Robinson and Ferrier, 2006) found that cognitive impairment was associated with number previous manic episodes, hospitalizations and length of illness. As with many studies in this area, poorly controlled samples was cited as an issue, although a recent and better controlled study (Lopéz-Jaramillo et al., 2010a) has supported this conclusion with clinical groups clearly matched on demographic variables including both medicated and unmedicated patients.
Such evidence has led to the development of the ‘staging model’ of bipolar disorder (Berk et al., 2007; Vieta et al., 2010) that argues for a clear clinical evolution of the condition over time, having strong implications for the importance of early intervention (Berk et al., 2010). Our results have implications both for early intervention and for cognitive rehabilitation, indicating that prompt and effective treatment may better preserve essential executive functions, while those patients with neurocognitive problems may be best treated by rehabilitation programmes that focus on their dysexecutive problems.
However, it is important to note that the data from this study, as with many other in the area, is purely cross-sectional and longitudinal studies will be needed to fully understand the relation between clinical evolution and cognitive impairment. This is particularly pertinent in bipolar disorder, owing to evidence suggesting that, in contrast to patients with schizophrenia for example, bipolar patients show an intact developmental cognitive trajectory but impairments that reflect worsening symptoms (Lewandowski et al., 2010). Furthermore, it is also not clear which cognitive impairments might fluctuate as the clinical picture alters and which might be relatively invariant. However, our results, along with those of Thompson et al. (2009) highlight the importance of looking at cognitive performance as an interacting system, rather than assuming the existence of individual components on the basis that they are represented by distinct numerical indices.
Bibliography
Arnone D, Cavanagh J, Gerber D, et al. Magnetic resonance imaging studies in bipolar disorder and schizophrenia: meta-analysis. Br J Psychiatry. 2009;195:194-201. [ Links ]
Arts B, Jabben N, Krabbendam L, et al. A 2-year naturalistic study on cognitive functioning in bipolar disorder. Acta Psychiatr Scand. 2011;123:190-205. [ Links ]
Baldo JV, Schwartz S, Wilkins D, et al. Role of frontal versus temporal cortex in verbal fluency as revealed by voxelbased lesion symptom mapping. J Int Neuropsychol Soc. 2006;12:896-900. [ Links ]
Balanzá-Martínez V, Rubio C, Selva-Vera G, et al. Neurocognitive endophenotypes (endophenocognitypes) from studies of relatives of bipolar disorder subjects: a systematic review. Neurosci Biobehav Rev. 2008;32:1426-38. [ Links ]
Berk M, Hallam K, Malhi GS, et al. Evidence and implications for early intervention in bipolar disorder. J Ment Health. 2010;19:113-26. [ Links ]
Berk M, Hallam KT, McGorry PD. The potential utility of a staging model as a course specifier: a bipolar disorder perspective. J Affect Disord. 2007;100:279-81. [ Links ]
Birn RM, Kenworthy L, Case L, et al. Neural systems supporting lexical search guided by letter and semantic category cues: a self-paced overt response fMRI study of verbal fluency. Neuroimage. 2010;49;1099-107. [ Links ]
Bora E, Yucel M, Pantelis C. Cognitive endophenotypes of bipolar disorder: a meta-analysis of neuropsychological deficits in euthymic patients and their first-degree relatives. J Affect Disord. 2009;113:1-20. [ Links ]
Daban C, Martínez-Arán A, Torrent C, et al. Cognitive functioning in bipolar patients receiving lamotrigine: preliminary results. J Clin Psychopharmacol. 2006;26:178-81. [ Links ]
Dittmann S, Hennig-Fast K, Gerber S, et al. Cognitive functioning in euthymic bipolar I and bipolar II patients. Bipolar Disord. 2008;10:877-87. [ Links ]
Ellison-Wright I, Bullmore E. Anatomy of bipolar disorder and schizophrenia: a meta-analysis. Schizophr Res. 2010;117:1-12. [ Links ]
Fountoulakis KN. An update of evidencebased treatment of bipolar depression: where do we stand? Curr Opin Psychiatry. 2010;23:19-24. [ Links ]
Glosser G, Gallo JL, Clark CM, et al. Memory encoding and retrieval in frontotemporal dementia and Alzheimer's disease. Neuropsychology. 2002;16:190-6. [ Links ]
Goswami U, Sharma A, Varma A, et al. The neurocognitive performance of drugfree and medicated euthymic bipolar patients do not differ. Acta Psychiatr Scand. 2009;120:456-63. [ Links ]
Gualtieri CT, Morgan DW. The frequency of cognitive impairment in patients with anxiety, depression, and bipolar disorder: an unaccounted source of variance in clinical trials. J Clini Psychiatry. 2008;69:1122-30. [ Links ]
Jamrozinski K. Do euthymic bipolar patients have normal cognitive functioning? Curr Opin Psychiatry. 2010;23:255-60. [ Links ]
Jamrozinski K, Gruber O, Kemmer C, et al. Neurocognitive functions in euthymic bipolar patients. Acta Psychiatr Scand. 2009;119:365-74. [ Links ]
Kempton MJ, Geddes JR, Ettinger U, et al. Meta-analysis, database, and metaregression of 98 structural imaging studies in bipolar disorder. Arch Gen Psychiatry. 2008;65:1017-32. [ Links ]
Kurtz MM, Gerraty RT. A meta-analytic investigation of neurocognitive deficits in bipolar illness: profile and effects of clinical state. Neuropsychology. 2009;23:551-62. [ Links ]
Lewandowski KE, Cohen BM, Ongur D. Evolution of neuropsychological dysfunction during the course of schizophrenia and bipolar disorder. Psychol Med. 2011;41:225-41. [ Links ]
López-Jaramillo C, Lopera-Vásquez J, Gallo A, et al. Effects of recurrence on the cognitive performance of patients with Bipolar I disorder: implications for relapse prevention and treatment adherence. Bipolar Disord. 2010;12:557-67. [ Links ]
López-Jaramillo C, Lopera-Vásquez J, Ospina-Duque J, et al. Lithium treatment effects on the neuropsychological functioning of patients with bipolar I disorder. J Clin Psychiatry. 2010;71:1055-60. [ Links ]
Moscovitch M. A neuropsychological model of memory and consciousness. En: Squire LR, Butters N, eds. Neuropsychology of memory. New York: Guilford; 1992. p. 5-22. [ Links ]
Palacio CA, García J, Arbeláez MP, et al. Validación de la entrevista diagnóstica para estudios genéticos (DIGS) en Colombia. Biomédica. 2004;24:56-62. [ Links ]
Ragland JD, Laird AR, Ranganath C, et al. Prefrontal activation deficits during episodic memory in schizophrenia. Am J Psychiatry. 2009;166:863-74. [ Links ]
Robinson LJ, Thompson JM, Gallagher P, et al. A meta-analysis of cognitive deficits in euthymic patients with bipolar disorder. J Affect Disord. 2006;93:105-15. [ Links ]
Robinson LJ, Ferrier IN. Evolution of cognitive impairment in bipolar disorder: a systematic review of cross-sectional evidence. Bipolar Disord. 2006;8:103-16. [ Links ]
Roca M, Martin-Santos R, Saiz J, et al. Diagnostic Interview for Genetic Studies (DIGS): inter-rater and test-retest reliability and validity in a Spanish population. Eur Psychiatry. 2007;22:44-8. [ Links ]
Sánchez-Moreno J, Martínez-Aran A, Colom F, et al. Neurocognitive dysfunctions in euthymic bipolar patients with and without prior history of alcohol use. J Clin Psychiatry. 2009;70:1120-7. [ Links ]
Sánchez-Moreno J, Martínez-Aran A, Tabarés-Seisdedos R, et al. Functioning and disability in bipolar disorder: an extensive review. Psychother Psychosom. 2009;78:285-97. [ Links ]
Shimamura AP. Memory and the prefrontal cortex. Ann N Y Acad Sci. 1995;769:151-9. [ Links ]
Shimamura AP, Janowsky JS, Squire LR. What is the role of frontal lobe damage in memory disorders? En: Levin HS, Eisenberg HM, Benton AL, eds. Frontal lobe function and dysfunction. New York: Oxford University Press; 1991. p. 173-95. [ Links ]
Taconnat L, Clarys D, Vanneste S, et al. Aging and strategic retrieval in a cued-recall test: the role of executive functions and fluid intelligence. Brain and Cognition. 2007;64:1-6. [ Links ]
Thompson JM, Gray JM, Crawford JR, et al. Differential deficit in executive control in euthymic bipolar disorder. J Abnorm Psychol. 2009;118:146-60. [ Links ]
Torres IJ, Boudreau VG, Yatham LN. Neuropsychological functioning in euthymic bipolar disorder: a metaanalysis. Acta Psychiatr Scand Suppl. 2007;434:17-26. [ Links ]
Vieta E, Reinares M, Rosa AR. Staging bipolar disorder. Neurotox Res. 2011;19:279-85. [ Links ]
Vita A, De Peri L, Sacchetti E. Gray matter, white matter, brain, and intracranial volumes in first-episode bipolar disorder: a meta-analysis of magnetic resonance imaging studies. Bipolar Disord. 2009;11:807-14. [ Links ]
Vgontzas AN, Kales A, Bixler EO. Benzodiazepine side effects: role of pharmacokinetics and pharmacodynamics. Pharmacology. 1995;51:205-23. [ Links ]
Wingo AP, Harvey PD, Baldessarini RJ. Neurocognitive impairment in bipolar disorder patients: functional implications. Bipolar Disord. 2009:11:113-25. [ Links ]
Wingo AP, Wingo TS, Harvey PD, et al. Effects of lithium on cognitive performance: a meta-analysis. J Clin Psychiatry. 2009;70:1588-97. [ Links ]
Wheeler MA, Stuss DT, Tulving E. Frontal lobe damage produces episodic memory impairment. J Int Neuropsychol Soc. 1995;1:525-36. [ Links ]