Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Colombiana de Psiquiatría
Print version ISSN 0034-7450
rev.colomb.psiquiatr. vol.45 no.3 Bogotá July/Sep. 2016
http://dx.doi.org/10.1016/j.rcp.2016.01.0020034-7450
Original article
Correlation Between Insight and Capacity to Consent to Research in Subjects With Bipolar Disorder Type I and Schizophrenia
Correlación entre la introspección y la capacidad para consentir a investigación de los sujetos con trastorno afectivo bipolar tipo I y con esquizofrenia
Carlos López-Jaramilloa,b,*, Chantal Aristizábal Toblerc, Constanza Ovalle Gómezd, Jaime Escobar Trianad
a Mood Disorders Program, Hospital Universitario San Vicente Fundación, Medellín, Colombia
b Research Group in Psychiatry (GIPSI), Department of Psychiatry, Universidad de Antioquia, Medellín, Colombia
c Hospital Central de la Policía Nacional, Universidad Nacional de Colombia, and Universidad El Rosario, Bogotá, Colombia
d Department of Bioethics, Universidad El Bosque, Bogotá, Colombia
*Corresponding author.
E-mail address: carloslopezjaramillo@gmail.com (C. López-Jaramillo).
ARTICLE INFO
Article history:
Received 16 January 2015
Accepted 16 January 2016
Available online 4 March 2016
ABSTRACT
Introduction: Schizophrenia and bipolar disorder type I (BD-I) can affect patient autonomy and capacity to consent to participate in research. Other variables associated with the autonomy of patients must be explored in order to improve the quality of the currently available tools.
Objective: To evaluate the relationship between insight and the capacity to consent to participate in research in patients with BD-I and schizophrenia.
Methods: A cross-sectional and longitudinal study was conducted with 120 subjects (40 subjects with schizophrenia, 40 with BD-I, and 40 healthy controls). The tools used were the Scale Assessment Insight-Expanded (SAI-E) and the MacArthur Competence Assessment Tool-Treatment (MacCAT-CR), which was first adapted culturally, and its validity and reliability assessed. The results obtained on each scale were compared and the association between them were evaluated.
Results: There is a direct correlation between the capacity to consent to research, measured using the MacCAT-CR tool, and the degree of insight, measured using the SAI-E scale, with an effect size of 1.3 for BD-I and 2.03 for schizophrenia.
Conclusions: The results suggest that there is a correlation between the degree of insight and the capacity to consent to research in subjects with schizophrenia and BD-I. Insight should therefore be included as a relevant variable to assess the capacity to consent, and future studies should include it when researching on or designing new tools which aim at a greater respect of patient autonomy.
Keywords: Schizophrenia, Bipolar disorder, Bioethics, Autonomy, Research.
RESUMEN
Introducción: La esquizofrenia y el trastorno afectivo bipolar tipo I (TBI) pueden afectar a la autonomía del paciente y su capacidad para consentir a la participación en proyectos de investigación. Otras variables asociadas con la autonomía de los pacientes deben ser exploradas con el fin de mejorar la calidad de los instrumentos actualmente disponibles.
Objetivo: Evaluar la relación entre la introspección y la capacidad de consentir a la participación en investigaciones en pacientes con TBI y esquizofrenia.
Métodos: Estudio longitudinal de corte transversal, en el que se incluyó a 120 sujetos (40 con esquizofrenia, 40 con TBI y 40 controles sanos). Las herramientas usadas fueron The Scale Assessment Insight-Expanded (SAI-E) y The MacArthur Competence Assessment Tool-Treatment (MacCAT-CR), que se adaptaron culturalmente antes de evaluar su validez y su confiabilidad. Se compararon los resultados obtenidos de cada escala y se evaluó la asociación entre estas.
Resultados: Hay correlación directa entre la capacidad de consentir a la participación en investigaciones, medida por la herramienta MacCART-CR, y el grado de introspección, medido por la escala SAI-E, con un tamaño de efecto de 1,3 para TBI y 2,03 para esquizofrenia.
Conclusiones: Los resultados indican que hay correlación entre el grado de introspección y la capacidad de consentir de los sujetos con esquizofrenia y los pacientes con TBI. Por lo tanto, la introspección debería incluirse como una variable relevante para evaluar la capacidad de dar consentimiento a la hora de participar en un estudio de investigación. Además, los estudios futuros deberían incluirlo para la investigación o diseño de nuevas herramientas que apunten a un mayor respeto de la autonomía de los pacientes.
Palabras clave: Esquizofrenia, Trastorno bipolar, Bioética, Autonomía, Investigación.
Introduction
Autonomy, understood as "the express capacity to impose norms or rules on oneself without the influence of external or internal pressure",1 is a basic principle of bioethics and constitutes one of the central aspects in determining an appropriate patient-doctor relationship.2,3
The health professional should favor and respect patients' autonomy with regards to the decisions they may take with reference to clinical practice and research, such as receiving specific treatment, submitting themselves to an intervention or participating in a research project.4 Difficulties arise in situations in which autonomy can be diminished or absent entirely, as is the case for patients with brain damage, psychological immaturity or mental illness.5 In these cases it is of vital importance to have evaluation tools available which allow the doctor to establish the patient's level of autonomy, and so strive to protect their rights.6
Scientific research involving human beings —in order to explore new diagnostic techniques and new treatment schemes— should only be carried out after free, express and informed consent has been given by the interested party.1 The Informed Consent (IC) process, both in its conception and its application, holds information, willfulness and the capacity of the subject to make decisions as its fundamental components,7 and represents the respect and guarantee of the free and autonomous participation of the subjects.8-10 Hence its unique and irreplaceable role in every research protocol involving human beings.11
In order that the patients' participation be truly free and voluntary, various conditions are required. Among these, and of some significance, is that the subject be capable of understanding the information that is being given to them, and of deciding based on their own reasoning.1 The information should be appropriate, easy to understand and include safeguards for retracting consent, in such a way that the subject is able to rescind their consent at any moment and for any reason12.
In vulnerable communities, in so far as the subjects may have decreased autonomy, and therefore lack the ability to decide for themselves,13 the need is even more apparent to be able to guarantee that all possible elements are in place to ensure the free, voluntary and autonomous choice of subjects that consent to participate in these research projects.12,14 It is taken that it is better to understand the ability to decide in relation to concrete situations, rather than as an over-arching fact: only in rare circumstances would a patient be completely incapacitated, the most common being patients who lack the capacity to make certain decisions, but are able to make others, or that have temporarily (as opposed to permanently) limited decision-making capacity, as is frequently the case with patients with chronic and persistent severe psychiatric disorders.10,15
Therefore assessing a subject's level of capacity is of great importance and interest, not only from a clinical and scientific perspective, but also —and perhaps more importantly— from a bioethical standpoint.16 In psychiatry it is expected that the problem of giving consent be more complicated, which has led to a growing interest in developing psychometric tools to assess the autonomy of the psychiatric patients involved in research projects.14,17 For this reason a number tools have been devised which allow the patient's decision-making capacity to be evaluated within a clinical and/or research context. These aim to respond to the complex problem of determining the subject's level of capacity, independent of the interest of the doctor or researcher,18 The MacCAT-CR tool is one of the most globally accepted methods for this very problem.16,19
One of the psychopathological findings that has been hypothetically associated with the capacity to consent has been the patients' level of insight. Some tools have already been designed to assess insight,20 and one of the most globally accepted, on account of its conceptual clarity and its proven validity, is Scale Assessment Insight-Expanded (SAI-E), which has been validated and widely used in Colombia.21
There are some studies which have studied this.16,22 Nevertheless, the relationship between the insight of the patient with regards to mental disorder and their ability to consent while suffering from the disorder is not completely clear, neither conceptually nor empirically.23 It is therefore necessary to determine if such a direct correlation exists, in which case a more comprehensive evaluation can be made of the patients who are being asked to consent. This would lead to a greater respect of the principle of autonomy,2 and offer the possibility to develope, in the future, a much more comprehensive psychometric tool for a more valid informed consent process, with better guarantees of respect for the autonomy of research subjects.24
Based on this, the objective of the present study is to establish the relationship between the capacity to consent to research and the level of insight of mental illness in adult patients with BD-I and schizophrenia, as determined by MacCAT-CR and SAI-E respectively.19,21 The starting hypothesis was that in patients with severe psychiatric disorders, there is a direct correlation between the level of insight and the ability to consent.
Methods
The study was observational and analytical, with crosssectional and longitudinal design. The design was crosssectional in the construct validity studies, as well as in the study to determine the relationship between the results obtained on the MacCAT-CR scale and the SAI-E scale,19,21 and longitudinal in the reliability study in which time measurements were taken.
Study Subjects
The target group consisted of 80 subjects that met the DSMIV diagnostic criteria25 for schizophrenia (n = 40) and bipolar disorder type I (n = 40), that were Spanish-speaking and that participated, or had been invited to participate, in any of the research studies carried out by the Psychiatric Research Group from the University of Antioquia (GIPSI) or the Mood Disorders Program of the San Vicente Fundación Hospital. A group of healthy volunteers (n = 40) were also included as control subjects who had no history of serious mental disorder. Inclusion criteria for all study groups were: being older than 18 and not being physically incapable of completing the study on account of unwillingness to participate, a state of extreme agitation or if the patient's state of consciousness was compromised.
Procedures and Tools
Subjects had to voluntarily agree to participate in the study, after an explanation of informed consent in which the goals of the research, the risks of participating were clearly explained and all doubts were resolved. Also, it was included the presence of witnesses. Subjects had the freedom to suspend, postpone or cancel the interview at any time. The ethics committee from the Faculty of Medicine at the Universidad de Antioquia and the Department of Bioethics from Universidad El Bosque evaluated and approved this study.
The study consisted of four stages: 1) validation and adaptation of the MacCAT-CR Tool,19 and analysis of face and content validity and of reliability of this tool; 2) application of the MacCAT-CR tool to the subjects with severe mental disorders and the control group; 3) application of the SAI-E scale21 to the same subject groups, and 4) analysis of the results and of the association of the results obtained on each tool (MacCAT-CR and SAI-E).
Cultural Adaptation of the MacCAT-CR Tool
Three translations of the MacCAT-CR tool were made, from English to Spanish, by three translators with prior knowledge of the tool. Subsequently each translation into Spanish was back-translated into English by a translator who had no prior knowledge of the tool. An interdisciplinary committee of 3 people —2 experts in bioethics and 1 translator— assessed the translations into English, or back-translations, and chose the version that was closest to the original.
The translation into Spanish from which the backtranslation was derived, was the most similar to the original tool (in English), and it was chosen by the interdisciplinary committee as the basis for the final text. Parts of the other available translations that were deemed more precise by the committee were integrated into this text, or modifications were made to which the translators consented when differences were identified between the two texts in English —the original tool and the selected back-translation. Likewise, some adaptations were made in order to make the tool understandable and ensure transcultural equivalence.
Analysis of Face and Content Validity of the MacCAT-CR Tool
The resulting Spanish version of the tool's cultural adaptation was then submitted to a group consisting of three psychiatrists with experience in bioethics, who were charged with reviewing each of the MacCAT-CR questions, with the aim of assessing their face and content validity.
The goal of this pilot test was to observe the comprehensibility and ambiguity of the texts, the presence of emotionally loaded questions, the time needed to fill out the tool, the need for training, and the ease of clarification and the frequency of responses. In relation to this point, if a question was answered in one direction more than 95% of the time, this indicated that the question needed revision. However, this did not happen for any of them.
Determining the Reliability of the MacCAT-CR Tool
In order to assess reliability, 20 subjects that met the diagnostic criteria for schizophrenia or BD-I were cited as possible candidates for participating in some of the research projects, as well as people that were not, or had not been, in psychiatric treatment. The MacCAT-CR tool was applied to the subjects by a psychiatrist, and one week later the same tool was reapplied to the same subjects. In order to determine the MacCATCR's reliability, test-retest reproducibility was measured and the intraclass correlation coefficient with its respective confidence interval of the 95% was calculated.
Application of the MacCAT-CR and SAI-E Tools
The MacCAT-CR tool was applied to the schizophrenia patients, the bipolar patients and the healthy subjects. Construct validity was determined by comparing the results of the three study groups. The SAI-E scale was applied to the group of subjects who were diagnosed with BD-I and schizophrenia to whom the Mac-CAT-CR tool had previously been applied. SAI-E scale was not necessary being applied to control group.
Statistical Analysis
In order to calculate the sample size, the formula for measuring reproducibility was employed using an error percentage of 5%, a test strength of 80%, an expected minimum intraclass correlation coefficient of .7 or > .8, and for two applications of the tool.26 The estimated sample size was 118 participants, and the decision was made to recruit 120 subjects.27
The demographic characteristics were described using the absolute frequency and percentage for the variable of gender and marital status. For the variables of age, schooling and time with disorder, the mean and standard deviation were used, the same as for the description of the points score for the MacCAT-CR and the SAI-E scale.
Group comparison was made using the χ2 test for the qualitative variables and the Kruskal-Wallis test for the quantitative variables. Capacity to consent and insight were compared among the subject groups with BD-I vs schizophrenia, subjects with BD-I vs control group subjects and subjects with schizophrenia vs. control group subjects, by using the Mann-Whitney U test. The subjects with severe mental disorders were classified into two groups based on whether or not the capacity to consent was present according to the results of the MacCAT-CR tool. These two groups, with and without the capacity to decide respectively, were then compared using the Mann-Whitney U test in the subjects with TBA-I and the subjects with schizophrenia. The effect size was calculated by assuming significant differences if d > .70.
The Spearman's rank correlation coefficient was calculated between the level of insight according to the SAI-E scale and the capacity to consent to research according to their level of understanding, reasoning and the MacCAT-CR's estimation. The scatter chart for the subject groups with severe mental illness (TBA-I and schizophrenia) was thus created.
Results
Sociodemographic Characteristics
When comparing the demographic characteristics of patients with BD-I, patients with schizophrenia and control subjects, significant differences are found in age (P < .001), marital status (P = .006) and time with the disorder (P = .013). In the schizophrenia group 73% were male versus 50% in the BDI group and 47% in the control group. However, there were not statistically significant differences. The sociodemographic characteristics of each group are shown in Table 1.
Comparative Findings of the MacCAT-CR Tool
Generally it may be observed that in the group of patients suffering from schizophrenia there was worse performance on the MacCAT-CR tool than in the group of patients with BD-I and the control group, with a statistical difference (P < .001) in all of the categories of the MacCAT-CR tool that were mentioned. Based on the results obtained using the MacCAT-CR tool, it was found that, while 47.5% of the subjects suffering from schizophrenia were considered incapable of consenting, only 15% of the subjects with BD-I were considered incapable of giving consent (Table 2).
Correlation of the MacCAT-CR and SAI-E Results
After evaluating the level of correlation between the results on the MacCART and SAI-E tools, the size effect was found to be > .70 in both groups: 1.3 in the group of subjects with BD-I (P = .015) and 2.03 in the group of subjects with schizophrenia (P < .001), which indicates the difference between the level of insight among the patients who were capable of consenting, obtained with the SAI-E scale, and the level of insight among the patients who were incapable of giving consent, obtained with the MacCAT-CR tool, with a larger effect size for schizophrenia (Table 3).
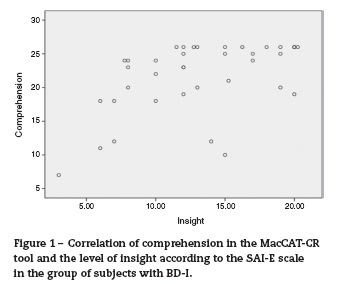
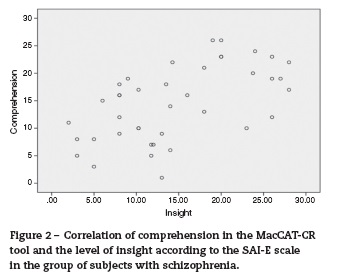
Figures 1 and 2 show the scatter chart for comprehension from the MacCAT-CR tool in correlation with the level of insight according to the SAI-E scale of the two subject groups with TBA-I and schizophrenia. Regarding the dimension of comprehension (i.e., understanding) in the two groups of subjects with severe mental disorders, there is a clear correlation between the level of comprehension in subjects with severe mental illness and the level of insight: the greater the insight, the greater the capacity to comprehend.
No clear relationship is observed between the level of appreciation and the level of insight for the group of patients with BD-I. However, these two aspects —appreciation and level of insight—do appear to be clearly related in the group of patients suffering from schizophrenia. A similar dispersion can be observed in both subject groups with severe mental illness, where there is correlation between reasoning and the level of insight; the greater the insight the greater the reasoning in both groups.
Discussion
The results of this study show that, for subjects with BD-I and schizophrenia, there is a direct correlation between the level of insight regarding their disease, and their capacity to consent in relation to giving informed consent to participate in research: subjects with a greater level of insight towards their mental disorder have a greater capacity to consent.28 These findings are consistent with similar studies carried out on these types of patients, not only for research but in the clinical area, with regards to accepting or refusing treatment for their disease.23,29 Various authors have already highlighted the importance of insight in severe mental disorders,30-33 not only as a factor to take into account when establishing the prognosis and evolution of the diseases,34 but also as a determining factor in understanding and establishing how the subject perceives their own disorder.35
The results also confirm the existence of differences in their capacity for informed consent for participating in research among the patients with severe psychiatric disorders and the healthy control subjects, as well as differences in the degree of compromise among the patients schizophrenia and BD-I. Finally, the findings show that the level of impairment of the patient's insight interferes with their capacity for informed consent and that both functions —insight and capacity to consent— might be interrelated superior mental processes, affected to different extents by the severity of the disorder.
It is important to highlight that the sociodemographic characteristics of the study population are similar to those of the patients who normally participate in research projects, so that these results can be easily extrapolated. When considering the demographic variables, it can be observed that there are no statistically significant differences in schooling between the three groups. Controlling for this variable is important, as it is a determining variable that could influence the patient's understanding or reasoning in relation to the tools used.
Although the authors acknowledge as a limitation of the study that psychopathological severity was not included in the data analysis,36 the length of time the patients have been diagnosed with the disorder for leads us to deduce that this sample population has a longstanding diagnosis as well as a significant level of severity. However, it would also be important for future research to evaluate the presence and severity of the symptoms of the disease, as the relationship between the symptoms and the capacity to give consent has already been.29,33
In order to evaluate the patient's capacity to consent, up until now the only tools available to us are not sufficient to guarantee the individual's completely autonomous decision, even though they do assess important aspects: the subject's comprehension, their appreciation with regards to the aim of the investigation, reasoning and the individual's final decision over whether or not to participate in the research.18,24 This study suggests that the factor that most determines the free choice and autonomy of patients with severe mental disorder, such as schizophrenia and bipolar disorder, is very probably directly linked with the subject's own experience with their disease and their level of insight. The findings of this study open up possibilities for researchers to include insight as a variable for a more comprehensive evaluation of the capacity to consent.
Subjects with low insight that wish to participate in research raise bioethical questions whose resolution demands a much more rigorous informed consent process.37 One of the most common dilemmas is whether it is acceptable that a person with a low perception of disease freely participates in a research project on a disease they do not think they have in the first place.38,39 In terms of capacity, this makes a "false positive": a subject who is considered capable of deciding when they really are deprived of such capacity".10,12 This process must avoid making vulnerable the autonomy of a patient who, with a diminished capacity to decide, runs the risk of failing to make a free and dignified decision.40
To the best of the knowledge of the authors, this is the first piece of research in which the relationships between insight and the capacity to give consent to participate in research were explored empirically. These findings allow us to make the following recommendations:
- Assessing the capacity to consent without taking into account insight is often insufficient and can impinge on the patient's autonomy. Therefore, both aspects should be assessed before considering candidates for patients with severe psychiatric disorders.
- If the results of the Insight show that it is decreased or affected in any way and, the patient still wants to participate, further warrants should included for this patients.
- If a patient with low insight and severe mental disorder is included, the consent should be obtained repeatedly throughout the study.
- The relationship between insight and capacity to consent should be explored in other mental disorders, as well as in other systemic diseases.
Conclusions
This study shows that there is a relationship between insight and the capacity to consent to participating in research in patients with severe mental disorders such as schizophrenia and BD-I, with a larger effect size for schizophrenia. Currently, there are no tools which take into account both elements —insight and capacity to consent— thus allowing that the principle of autonomy of the patients with mental disorders is more respected by researchers and clinicians.2,18,24 Considering the difficulties in the capacity to consent of the patients with schizophrenia and BD-I along with the findings of this study, which highlight the crucial role of insight to develop the consenting capacity, the next step must be to develop or improve a psychometric tool which includes insight as a variable. Indeed, assessing the capacity to consent without taking insight into account is insufficient, and runs the risk of infringing on the subject's autonomy, and therefore their dignity and freedom.1
Conflicts of interests
None of the authors have a conflict of interests to declare.
Acknowledgements
The authors thank to the Research Department of El Bosque University, CODI-University of Antioquia and the Sustainability Strategy for supporting this project.
REFERENCES
1. Beauchamp T, Childress J. Principios de ética biomédica. Barcelona: Masson; 1999. [ Links ]
2. Dekkers W. Autonomy and dependence: chronic physical illness and decision-making capacity. Med Health Care Philos. 2001;4:185-92. [ Links ]
3. Dickenson D. Decision-making competence in adults: a philosopher's viewpoint. Adv Psych Treat. 2001;7:381-7. [ Links ]
4. Kallert T. Coercion in psychiatry. Curr Opin Psychiatry. 2008;21:458-9. [ Links ]
5. Eastman N, Starling B. Mental disorder ethics: theory and empirical investigation. J Med Ethics. 2006;32:94-9. [ Links ]
6. Shore D. Ethical issues in schizophrenia research: a commentary on some current concerns. Schiz Bull. 2006;32:26-9. [ Links ]
7. Beauchamp T. Los fundamentos filosóficos de la ética en psiquiatría. La ética en psiquiatría. 1.ª ed. Madrid: Triacastela; 2001. p. 35-56. [ Links ]
8. Richman KA. Ethics and the Metaphysics of Medicine: reflections on health and beneficence. Cambridge, Massachusetts, London: The MIT Press; 2004. [ Links ]
9. Robertson M, Walter G. The ethics of psychiatric diagnosis. Psych Ann. 2007;37:792-7. [ Links ]
10. Simón P. Dificultades para valorar la competencia. Barcelona: Ponencia en las Jornadas Víctor Grífols i Lucas "Aproximació al problema de la competència del malalt"; 2006. [ Links ]
11. Cohen BJ, McGarvey EL, Pinkerton RC, Kryzhanivska L. Willingness and competence of depressed and schizophrenic inpatients to consent to research. J Am Acad Psychiatry Law. 2004;32:134-43. [ Links ]
12. Simón P, Barrio I. ¿Quién decidirá por mí? Madrid: Triacastella; 2004. [ Links ]
13. Husted JR. Insight in severe mental illness: implications for treatment decisions. J Am Acad Psychiatry Law. 1999;27:33-49. [ Links ]
14. Sánchez J. El consentimiento informado en psiquiatría. Madrid: Díaz de Santos; 2002. [ Links ]
15. Jones G. Informed consent in chronic schizophrenia? Br J Psychiatry. 1999;167:565-8. [ Links ]
16. Appelbaum PS. Decisional capacity of patients with schizophrenia to consent to research: taking stock. Schiz Bull. 2006;32:22-5. [ Links ]
17. Moser DJ, Reese RL, Hey CT. Using a brief intervention to improve decisional capacity in schizophrenia research. Schiz Bull. 2006;32:116-20. [ Links ]
18. Sturman ED. The capacity to consent to treatment and research: a review of standardized assessment tools. Clin Psychol Rev. 2005;25:954-74. [ Links ]
19. Grisso T, Appelbaum PS, Hill-Fotouhi C. The MacCAT-T: a clinical tool to assess patients' capacities to make treatment decisions. Psychiatr Serv. 1997;48:1415-9. [ Links ]
20. Yen C, Chen C, Yeh M, Yen J, Ker J, Yang S. Comparison of insight in patients with schizophrenia and bipolar disorder in remission. J Nerv Ment Dis. 2002;190:847-9. [ Links ]
21. Navarro F, Holguin J, Cano J, Cardeño C, Gómez J, Jiménez K, et al. Validación de la versión para Colombia de la Escala de Evaluación de Introspección Expandida (SAI-E) en sujetos con trastornos afectivos y psicóticos. Rev Colomb Psiquiatr. 2008;37:330-43. [ Links ]
22. Appelbaum PS, Grisso T, Frank E, Donnell SO, Kupfer DJ. Competence of Depressed Patients for Consent to Research. Am J Psychiatry. 1999;156:1380-4. [ Links ]
23. Misra S, Ganzini L. Capacity to consent to research among patients with bipolar disorder. J Affect Disord. 2004;80:115-23. [ Links ]
24. Chiswick D. Test of capacity has little practical benefit. BMJ. 2005;331:1469-70. [ Links ]
25. DSM-IV: Diagnostic and Statistical Manual of mental Disorders. Washington: American Psychological Association; 1994. [ Links ]
26. Walter S, Eliasziw M, Donner A. Sample size and optimal designs for reliability studies. Stat Med. 1998;17:101-10. [ Links ]
27. Machin D. Sample size tables for clinical studies. 2nd ed. Oxford: Blackwell Science; 1997. [ Links ]
28. Manson N, O'Neill O. Rethinking Informed Consent in Bioethics. Cambridge: Univerity of Cambridge; 2007. [ Links ]
29. Braw Y, Sitman R, Sela T, Erez G, Bloch Y, Levkovitz Y. Comparison of insight among schizophrenia and bipolar disorder patients in remission of affective and positive symptoms: analysis and critique. Eur Psychiatry. 2012;27:612-8. [ Links ]
30. Amador X. Assessment of Insight in Psychosis. Curr Opin Psychiatry. 2007;9:512-20. [ Links ]
31. Marková I, Berrios G. Insight in clinical psychiatry revisited. Compr Psychiatry. 1995;36:367-76. [ Links ]
32. Pini S, Cassano GB, Dell'Osso L, Amador XF. Insight into illness in schizophrenia, schizoaffective disorder, and mood disorders with psychotic features. Am J Psychiatry. 2001;158:122-5. [ Links ]
33. Smith T, Hull J, Goodman M, et al. The relative influences of symptoms, insight, and neurocognition on social adjustment in schizophrenia and schizoaffective disorder. J Nerv Ment Dis. 1999;187:102-8. [ Links ]
34. McEvoy J, Apperson L, Appelbaum P, Ortlip P, Brecosky J, Hammill K, et al. Insight in schizophrenia. Its relationship to acute psychopathology. J Nerv Ment Dis. 1989;177:43-7. [ Links ]
35. Villagrán J, Luque R. Psicopatología descriptiva: nuevas tendencias. Madrid: Trotta; 2000. p. 389-419. [ Links ]
36. Smith T, Hull J, Santos L. The relationship between symptoms and insight in schizophrenia: a longitudinal perspective. Schizophr Res. 1998;33:63-7. [ Links ]
37. Van Putten T, Crumpton E, Yale C. Drug refusal in schizophrenia and the wish to be crazy. Arch Gen Psychiatry. 1976;33:1443-6. [ Links ]
38. O'Neill O. Some limits of informed consent. J Med Ethics. 2003;29:4-7. [ Links ]
39. Dunn LB, Candilis PJ, Roberts LW. Emerging empirical evidence on the ethics of schizophrenia research. Schizophr Bull. 2006;32:47-68. [ Links ]
40. Welie S, Berghmans R. Inclusion of patients with severe mental illness in clinical trials: issues and recommendations surrounding informed consent. CNS Drugs. 2006;20:67-83. [ Links ]