Introduction
There are multiple metabolic enzymes involved in xenobiotic metabolism. The cytochrome P450 (CYP) isoenzymes are the most important group of functionalizing enzymes or what was previously called Phase I metabolism. The CYP isoenzyme 3A4 (CYP3A4) is the most important in hepatic metabolism and is found in the small intestine.1 As a matter of fact, CYP3A4 is a fundamental component of first-pass metabolism of many drugs, which refers to the substantial decrease in bioavailability of some drugs when administered orally, as opposed to intravenously (IV). (2 Metabolism in the intestine and the first time through the liver constitute first-pass metabolism. Another CYP isoenzyme from the same family, 3A5 (CYP3A5), is highly homologous with CYP3A4 and appears to metabolize many of the same substrates as CYP3A4, and plays a key role in drugs metabolized in the kidneys. However, for the majority of drugs extensively metabolized by CYP3A4, the contribution of CYP3A5 to their total metabolism is believed to be small; the majority of Caucasian do not express CYP3A5. (3
The concentration-to-dose (C/D) ratio is a measure of the ability to eliminate the drug and is influenced by genetic, personal and environmental factors, and can be used to personalize the prescription of psychiatric medications in psychopharmacology. Inducers decrease the C/D ratio and inhibitors increase the C/D ratio. In comparing individuals taking the same drug, a very low C/D ratio indicates an individual with very fast metabolism, while a very high C/D ratio indicates one with very slow metabolism. Each drug is different and unique, and has its own normal ranges for C/D ratios determined by its own bioavailability and elimination from the body.
Supplementary tables S1 to S7 describe pharmacokinetics, including metabolism and therapeutic drug monitoring (TDM), with an emphasis on the C/D ratios of 7 drugs. The pharmacokinetics of these drugs are described in order to understand the 2 cases reported in this article and how they can reflect high sensitivity to CYP3A4 induction and normal metabolism in other CYPs. The pharmacokinetics of carbamazepine is described in Supplementary Table S1, (4-13 diazepam in Supplementary Table S2, (11,14-20 quetiapine in Supplementary Table S3, (9,11,20-30 risperidone in Supplementary Table S4, (31-39 paliperidone in Supplementary Table S5, (11,26,31,40-46 olanzapine in Supplementary Table S6, (26,47-54 and clozapine in Supplementary Table S7. (55-58
There is general agreement in the literature that carbamazepine (Supplementary Table S1) and quetiapine (Supplementary Table S3) are dependent on CYP3A4 for their metabolism. A patient with sensitivity to CYP3A4 induction should have very high metabolism and low C/D ratios for these 2 drugs. There is general agreement that olanzapine (Supplementary Table S6) and clozapine (Supplementary Table S7) are not dependent on CYP3A4 for their metabolism, although they are sensitive to CYP1A2 (and possibly glucuronidation) induction during carbamazepine treatment. A patient with sensitivity to CYP3A4 induction should have normal clozapine and olanzapine metabolism and, when taking carbamazepine, should have metabolism similar to the average patient taking phenytoin or carbamazepine and should have similar C/D ratios. A recent comprehensive and systematic review39 of risperidone pharmacokinetics describes CYP2D6 as the main metabolic enzyme for risperidone in normal patients, but in patients taking inducers CYP3A4 becomes the most important metabolic pathway. Based on the literature, (17,26,31-45 this article proposes that in patients taking potent CYP3A4 inducers such as carbamazepine or phenytoin, diazepam (Supplementary Table S2), risperidone (Supplementary Table S5) and paliperidone (Supplementary Table S6) become more dependent on CYP3A4 for their metabolism. Therefore, a patient with sensitivity to CYP3A4 induction should have very high metabolism and low C/D ratios for these other 3 drugs: diazepam (Supplementary Table S2), risperidone (Supplementary Table S5), and paliperidone (Supplementary Table S6).
The following 2 rare cases describe 2 patients needing extremely high doses of CYP3A4 medications to reach and maintain therapeutic concentrations. The high metabolic ability of these patients was demonstrated by the low C/D ratios of several CYP3A4 drugs.
Methods
TDM
These 2 cases contain TDM information that was collected for clinical purposes many years ago using the commercial laboratory available at the psychiatric hospital. All serum levels were taken as trough concentrations (early AM before taking medications) after reaching steady state and measured at the commercial laboratory contracted by the psychiatric hospital.
C/D Ratio
The interpretation of clozapine and risperidone C/D ratios has been described previously. (55 These 2 drugs, like the majority of drugs used in neuropsychopharmacology, follow linear kinetics. A linear relationship exists between typical doses and plasma concentrations. This means that the relationship between concentration and dose is stable; it does not change with different doses and concentrations, and the drug C/D ratio is constant in the same patient as long as there are no changes in environmental or personal variables.
The C/D ratio of carbamazepine metabolism is described in Supplementary Table S1, (11,12 diazepam in Supplementary Table S2, (4,16-19, quetiapine in Supplementary Table S317,28-30, risperidone in Supplementary Table S4, (31,39 paliperidone in Supplementary Table S5, (17,45,46 olanzapine in Supplementary Table S6, (26,47,49-54 and clozapine in Supplementary Table S7. (55
Case Presentations
Case 1
A Caucasian male was followed for more than 4 years from ages 30 to 34 years at a US state psychiatric hospital. His initial weight was 85 kg. He smoked 10 cigarettes per day. His psychosis started when he was 12 years old and had been refractory to treatment for many years at the time of admission. After signing a consent form, he was identified by genotyping as a CYP2D6 extensive metabolizer (EM) and a poor metabolizer for CYP3A5. (42
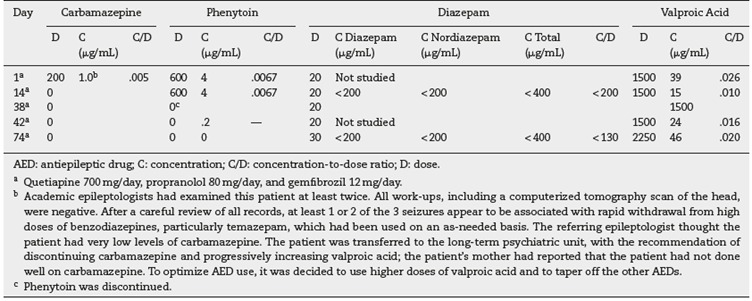
The patient had 3 seizures of unknown origin in the 3 months prior to initiating treatment in the state psychiatric hospital (Table 1, footnote b). At the time of the initial psychiatric evaluation, he was on 4 AEDs: carbamazepine, phenytoin, diazepam and valproic acid (VPA), which had been progressively added by academic epileptologists. The blood levels of all 4 of these medications were subtherapeutic (Table 1). After the discontinuation of carbamazepine, phenytoin and diazepam, the patient was stabilized on a very high VPA dose of 5250 mg/day. This dose corresponds to 62.5 mg/kg/day, which is higher than the upper range of the highest recommended maintenance dose (30-60 mg/kg/day). (11
Table 1 Case 1. Daily doses (mg/day), concentrations and C/D ratios of AEDs during the initial part of admission.
Undetectable Concentrations of Diazepam as an AED
Even though he was taking a relatively high dose of oral diazepam, up to 30 mg/day, his diazepam and nordiazepam trough levels were undetected. The laboratory recommended a total level of 500-2000 ng/mL, calculated by adding diazepam and nordiazepam. The last undetectable diazepam level during the use of diazepam for AED treatment was measured 30 days after phenytoin discontinuation (Table 1).
Need of High Doses of Diazepam for Sedation Due to Rapid Metabolism which Normalized 1.5 Years Later
Supplementary Table S8 describes the diazepam response of this patient and Supplementary Table S9 shows the administration of 170 mg in 2 days led to a successful dental visit. The same dose of 170 mg in 2 days was successfully repeated again for the second and third dental visits, respectively one week and 2 weeks later. After each visit the diazepam was tapered off until the standard dose of 30 mg/day was reached.
As the blood levels of diazepam at 30 mg/day were undetectable, poor compliance was considered, but this was unlikely since: a) the nurses were very experienced in dealing with people who "cheek" medications; b) the patient stayed in the low-stimuli unit for aggressive patients, most of the time by himself with no other patients, and c) the unit was closely supervised by at least 3 staff members. The patient had been compliant with medications and had not demonstrated worrisome behaviors related to his medications. After excluding non-compliance, 2 explanations were possible: a) the patient was not absorbing diazepam (unlikely since oral diazepam is easily absorbed), and b) the patient was breaking down diazepam very rapidly during first-pass metabolism or later on during liver metabolism. To test these explanations, the guardian approved a pharmacokinetic study after IM diazepam was planned in order to bypass the effects of absorption and first-pass metabolism, which is described in Supplementary Table S9. The patient was taking the scheduled oral 30 mg/day which provided repeated undetectable TDM levels; he received 30 mg IM of diazepam early in the morning and repeated concentrations were measured. Based on this study (see footnote an in Supplementary Table S9), diazepam clearance was estimated to be more than double the normal. (59 It is remarkable that this diazepam clearance of double the normal was demonstrated 4 months after discontinuation of carbamazepine and 3 months after discontinuation of phenytoin.
Supplementary Table S8 describes later diazepam responses. Figure 1 tries to provide a representation of diazepam metabolism according to the temporal changes. The patient was an extremely fast metabolizer of diazepam at the beginning when he was taking phenytoin, but probably normalized 1.5 years after phenytoin discontinuation.
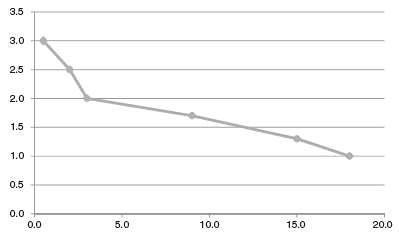
Figure 1 Case 1: estimation of diazepam metabolism after months of phenytoin discontinuation. Vertical axis represents diazepam metabolism, with normal metabolism represented by 1.0 and 3.0 representing a metabolism 3 times faster. The horizontal axis represents the number of months after phenytoin discontinuation. Diazepam metabolism was estimated to be: a) 3.0 for undetectable diazepam levels with 30 mg/day (17 days after phenytoin discontinuation); b) 2.5 for needing 170 mg in 2 days to go to the dentist 3 times (2 months after phenytoin discontinuation); c) 2.0 for an IM study demonstrating a diazepam clearance twice the normal rate (3 months after phenytoin discontinuation); d) 1.7 for a dose of 100 mg that in 2 days became too high (9 months after phenytoin discontinuation); e) 1.3 for a dose of 60 mg which in 1 h controlled agitation but led to signs of diazepam intoxication (15 months after phenytoin discontinuation), and f) 1 for a dose of 20 mg which controlled agitation with no signs of intoxication (18 months after phenytoin discontinuation).
Very Low Quetiapine C/D Ratio That Increased After Phenytoin Discontinuation
Supplementary Table S10 describes quetiapine metabolism. (29,30,60 In summary, the patient was an extremely fast metabolizer of quetiapine at the beginning when he was taking phenytoin, but his metabolism decreased after phenytoin discontinuation. This pattern is suggested by: a) a C/D ratio 10 times lower than normal during phenytoin treatment; b) a C/D ratio approximately 8 times lower than normal when phenytoin was discontinued, and c) C/D ratios 2.6 to 6.5 times lower than normal during the 3 to 5 months after phenytoin discontinuation.
Very High Carbamazepine Doses in the Past
A previous discharge summary suggested that the patient needed high doses of carbamazepine (1500-2000 mg/day) to reach therapeutic levels, which is much higher than the typical dosage. Table 1 provides a low carbamazepine C/D ratio, but it is seriously limited because the carbamazepine dose was being discontinued.
Normal Olanzapine C/D Ratios and Partial Response to Low Doses
Based on a review of available records and collateral information from the patient's mother, olanzapine appeared to be the only atypical antipsychotic not tried. A dose of 5 mg/day of olanzapine was subsequently added to his quetiapine. Two weeks after the addition of olanzapine, mild improvement was observed in his formal thought disturbance leading to transfer from the low-stimuli unit to the long-term unit (Supplementary Table S11, footnote a). Supplementary Table S11 summarizes olanzapine C/D ratios of 26 concentrations over more than 2 years, which provided a mean C/D ratio of 1.6 ng/mL/mg/day, which is within the normal range for a smoker. In conclusion, the patient had normal olanzapine metabolism and had a partial response to low olanzapine doses including an initial stabilization on 5 mg/day.
Normal Clozapine C/D Ratios
Clozapine was started by adding it to olanzapine. Olanzapine was completely discontinued after an episode of clozapine intoxication secondary to an upper respiratory infection. (61 The patient was stabilized on clozapine 700-800 mg/day and finally discharged from the hospital on 700 mg/day after 14 months on clozapine and more than 4 years of admission. There were 11 clozapine concentrations on 700 or 800 mg/day providing a mean clozapine C/D of 0.80 ng/mL/mg/day, which indicates normal metabolism for a male smoking 10 cigarettes/day (Supplementary Table S12).
Summary of CYP Metabolism
The patient appeared to be a normal metabolizer of olanzapine and clozapine, which are mainly dependent on CYP1A2. On the other hand, he appeared to have an extremely high ability to metabolize carbamazepine, quetiapine and diazepam when a CYP3A4 inducer was present in prior months, suggesting that he was very sensitive to the effects of CYP3A4 inducers.
Effects of CYP3A4 Induction Appeared to Last for Many Months
The effect of CYP3A4 inducers appeared to last many months since his quetiapine metabolism did not normalize for 3 months after stopping phenytoin. Diazepam was undetectable in the presence of phenytoin and continued to be metabolized very quickly 2 months after the discontinuation of phenytoin, when the IM diazepam study was conducted. The response to diazepam used for sedation suggested that diazepam would have normalized 1.5 years after the discontinuation of phenytoin (Supplementary Table S8 and Figure 1).
Extreme Sensibility to Akathisia
Supplementary Table S12 shows that the patient appeared to be extremely sensitive to akathisia on quetiapine and olanzapine. First, the akathisia became very evident with a quetiapine peak concentration that corresponded approximately to less than 225 mg/day according to the multicenter study, (29 and second, he developed akathisia with an olanzapine dose of 2.5 mg/day, when propranolol was decreased to 30 mg/day. The presence of akathisia with extremely low doses of these two antipsychotics not prone to cause akathisia suggests that the patient had a pharmacodynamic abnormality, possibly genetic. He developed akathisia whenever the antipsychotics reached more than low serum concentration. He developed no akathisia on clozapine.
Case 2
On a patient's third of 8 admissions to a US state psychiatric hospital, the senior author was consulted about high doses of psychiatric medications. The patient was a Caucasian male who was 28 years old at the time. He had schizoaffective disorder; was morbidly obese, weighing 191.4 kg (422 lbs); had a height 2.18 m (86 inches), and a BMI of 40.
When not in the hospital, he smoked 2 packs of cigarettes per day. His psychopharmacologic regimen included carbamazepine extended-release 2800 mg/day during the first 3 admissions. He was treated with very high doses of risperidone, olanzapine or paliperidone. Table 2 describes the doses, concentrations and C/D ratios during the 5 admissions. The C/D ratios of carbamazepine, risperidone, paliperidone, olanzapine and valproate are reviewed. The mother and guardian of this patient signed a written consent to publish the case.
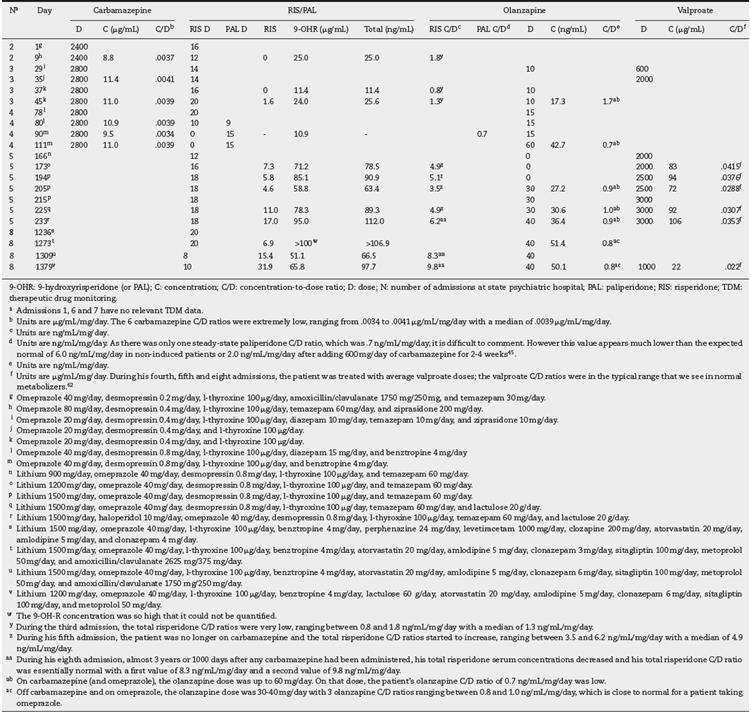
Table 2 Case 2. Daily doses (mg/day), concentrations and C/D ratios during the patient’s eight admissions.
Very High Carbamazepine Dose and Low Carbamazepine C/D Ratio
During his first 3 admissions the daily carbamazepine dose ranged between 2400 and 2800 mg/day (Table 2). These very high doses were required to get the therapeutic range of 4-12 µg/mL, as recommended by the laboratory. The 6 carbamazepine C/D ratios were extremely low (Table 2, footnote b).
Low Total Risperidone C/D Ratios During Carbamazepine Treatment Normalized >1 Year After Discontinuation
During these 4 admissions the patient was treated with up to 20 mg/day. The third admission was contaminated by the induction of carbamazepine, which provided very low total risperidone C/D ratios (Table 2, footnote b) with a median of 1.3 ng/mL/mg/day.
During his fifth admission, the patient was no longer on carbamazepine and the total risperidone C/D ratios started to increase with a median of 4.9 ng/mL/mg/day (Table 2, footnote z). During his eighth admission, almost 3 years or 1000 days after any carbamazepine had been administered, he developed akathisia with the very high risperidone dose of 20 mg/day and with extremely high serum 9-OH-R concentrations that could not be quantified. A major risperidone dose decrease was recommended. After a decrease to a normal risperidone dose of 8 mg/day, the akathisia disappeared. His total risperidone serum concentrations decreased and his total risperidone C/D ratio was essentially normal (Table 2, footnote aa). It appears that the treating physician in the community did not realize that it was okay to treat this patient with 20 mg/day of risperidone when he was on carbamazepine, although this dosage was too high for this patient without the inductive effects of carbamazepine.
Very Low Paliperidone C/D Ratio
As there was only one steady-state paliperidone C/D ratio, it is difficult to comment (Table 2, footnote d), but it is much lower than the expected normal ratio. (45
Normal Olanzapine C/D Ratio for Carbamazepine Induction
On carbamazepine (and omeprazole), the olanzapine dose was up to 60 mg/day. On that dose, the patient's olanzapine C/D ratio was low (Table 2, footnote ab). Off carbamazepine and on omeprazole, the olanzapine dose was 30-40 mg/day with 3 olanzapine C/D ratios, which is close to normal for a patient taking omeprazole (Table 2, footnote ac).
Normal Valproate Dose and C/D Ratio
During his fourth and fifth admissions, the patient was treated with average valproate doses11; the valproate C/D ratios (Table 2, footnote f) were in the typical range that we see in normal metabolizers. (62
Summary of CYP Metabolism
After taking into account the carbamazepine induction, the patient appeared to be normal for metabolizing olanzapine, which is mainly dependent on CYP1A2. He also appeared to be normal for valproate metabolism, which is mainly metabolized by UGTs and ß-oxidation, with a minor component of CYP2C19. (62 In the absence of carbamazepine, he had normal risperidone metabolism, but in the presence of carbamazepine he tolerated extremely high doses of risperidone, up to 20 mg/day. He also had an extremely high ability to metabolize carbamazepine. Therefore, he appeared to be very sensitive to the effects of CYP3A4 inducers.
Effect of CYP3A4 Induction Appeared to Last for Many Months
The effect of CYP3A4 inducers appeared to last many months since his risperidone metabolism did not normalize during his fourth admission, separated by 50 days from the third admission when he was on carbamazepine. Risperidone metabolism had reached a normal rate during the fifth admission, which was 3 years after the discontinuation of carbamazepine (Figure 2).
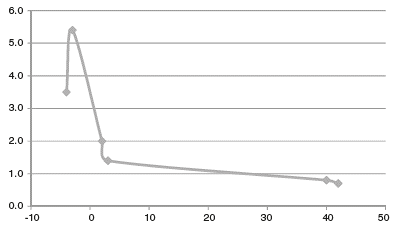
Figure 2 Case 2: risperidone metabolism after months of carbamazepine discontinuation. Vertical axis represents risperidone metabolism calculated using total risperidone concentration-to-dose ratios, where normal metabolism is represented by 1.0 and 3.0 represents a metabolism 3 times faster. The horizontal axis represents months before and after carbamazepine discontinuation, which is considered 0.
Discussion
Case 1
CYP Metabolism: A Circumscribed High CYP3A4 Metabolic Capacity Which Took More Than 1 Year to Normalize
The patient metabolized olanzapine and clozapine normally, which suggested normal metabolism for CYP1A2. On the other hand, he appeared to be very sensitive to CYP3A4 inducers. In normal metabolizers, the effects of CYP3A4 inducers are assumed to last only 3 weeks, (10 but in his case, they appear to have lasted for months, normalizing only after more than 1 year.
A Previously Published High VPA Metabolism Only on the Valproate Formulation
Case 1 received VPA for seizures and schizophrenia and had >50 VPA concentrations in 4 years. A high dose of 5,250?mg/day of VPA concentrate was prescribed for years, but this dose led to intoxication when switched to the enterocoated divalproex sodium formulation, requiring a normal dose of 2000 mg/day. (62
Akathisia on Antipsychotics Except for Clozapine
The patient had an unusual lack of tolerance to antipsychotic blockade. This was supported by the history of akathisia on typical antipsychotics reported by the mother and the documentation in several discharge summaries. Double-blind studies suggest that quetiapine does not cause more EPS than placebo63 and that akathisia is very rare. (64 However, this patient had akathisia on quetiapine with blood levels corresponding to a very low dose of 225 mg/day. He also developed akathisia when propranolol was decreased despite a very low olanzapine dose (2.5 mg/day). On the other hand, the patient tolerated clozapine without akathisia, not surprisingly, since clozapine does not cause akathisia or, if it does, it is extremely rare. (65
Case 2
A Circumscribed High CYP3A4 Metabolic Capacity
This patient appeared to be very sensitive to CYP3A4 inducers as indicated by very low C/D ratios for carbamazepine, risperidone and paliperidone. This patient had the highest dose ever seen in a carbamazepine patient and in a risperidone patient by the senior writer. In normal metabolizers, the effects of CYP3A4 inducers are assumed to last only 3 weeks10 but with him, risperidone metabolism lasted for many months and was definitively normal 3 years after discontinuing carbamazepine (Figure 2). It was unfortunate that his outpatient psychiatrists did not realize that prescribing 20 mg/day of risperidone was no longer safe in this patient after stopping carbamazepine.
Limitations
Case reports have major limitations, but also have a long tradition of generating testable hypotheses. (66 Moreover, these two patients were followed for years with repeated TDMs from multiple drugs. There are no similar published cases of patients taking these astronomic doses of carbamazepine, diazepam or risperidone, so it is safe to think that these types of patients with great sensitivity to CYP3A4 induction are extremely rare. The senior author found only these two cases in a 20-year span and, more importantly, it took him that long to understand the pharmacological mechanisms that may explain these high doses.
In these two cases, low C/D ratios during treatment with potent inducers such as carbamazepine and phenytoin are considered to be signs of fast metabolism that normalized when the inductive effects disappeared. Low C/D ratios can also be explained by poor compliance. However, these two inpatients were monitored by nurses very experienced with poor compliance and had low C/D ratios with some drugs but not with others, making it unlikely that the pattern was explained by a patient who decided to use selective poor compliance only with drugs metabolized by CYP3A4. Low C/D ratios can be also explained by poor oral absorption, but these patients did not have medical disorders associated with poor gastrointestinal absorption; these disorders tend to be associated with generalized rather than selective poor absorption of specific drugs. The diazepam IM study in Case 1 demonstrated that the patient had fast metabolism not only with oral administration but also with IM administration.
Prior Published Cases Using High Doses and Induction
The literature describes only 4 other inpatients with repeated TDM that demonstrate the need of very high doses of medications during induction using metabolic pathways other than CYP3A4. To get therapeutic levels in these other 4 patients sensitive to induction by 1) valproate, 1300 mg/day of clozapine was needed, (672) phenytoin and phenobarbital, 1600 mg/day of lamotrigine was needed, (283) valproate for its own metabolism, up to 10,500 mg/day of valproate was needed, (62 and 4) in another, up to 4000 mg/day of valproate was needed. (62 Case 1 in this article is unique because he appeared to be very sensitive to CYP3A4 induction and valproate auto-induction. (62
Of the 7 psychiatric drugs reviewed in this article (Supplementary Tables S1-S7), there are some published cases of high doses of carbamazepine, clozapine and olanzapine that provide at least one TDM measure, but few of the authors consider the possibility that induction was accounting for the high doses. An article in German68 described 41 patients on carbamazepine monotherapy who were selected for high serum carbamazepine concentrations (>9 µg/mL) or for a plan to increase the dose. The article figure provided one concentration around of 7 µg/mL in a patient taking around 3,250 mg/day, which allowed the calculation of a C/D ratio of 0.002 µg/mL/mg/day, which is highly compatible with a patient very sensitive to carbamazepine auto-induction as long as the patient was compliant with carbamazepine high dosing and prior carbamazepine TDMs, if they existed, were compatible with progressive auto-induction. The German authors did not comment on carbamazepine auto-induction as an explanation for the extremely high dosing for this patient68. A few patients have been described69-73 needing clozapine doses >900 mg/day or with clozapine C/D ratios indicative of the need for these high doses. Some, but not all, of the authors provided details verifying the absence of potent inducers such as carbamazepine, phenobarbital or phenytoin; in most cases the authors proposed that patients may have unusual CYP1A2 genetic variations. (69-73 A study of 50 patients with olanzapine doses >20 mg/day provided TDM and described 4 patients taking doses >60 mg/day but did not provide detail on co-medications of these 4 patients, although 3 of the 50 patients were taking AEDs. (74
A literature search identified no case report of high doses of risperidone or paliperidone but some case reports of high doses of diazepam75 and quetiapine, (76-80 apparently in the absence of CYP3A4 potent inducers but no TDM data was presented.
Finally, the possibility of induction contributing to very high caffeine doses was described in a caffeine study without TDM. (81 Caffeine is metabolized by CYP1A2 and this study reported that 3 of 265 long-term psychiatric patients had extremely high caffeine intake (>1499 mg/day). All three patients were under the influence of 2 inducers since they were smokers taking another CYP1A2 inducer, omeprazole. (81
Pharmacological Speculation That Possibly Explains These Two Very Unusual Patients
The 2 patients metabolize CYP3A4 drugs very rapidly. Four previously published cases28,62,67 describe very fast metabolism for drugs dependent on other metabolic pathways. There are no other published cases with high doses of psychiatric drugs associated with high sensitivity to induction. Although induction is not well understood, it appears to be mainly regulated by the so-called nuclear receptors which include: the constitutive androstane receptors (CAR), the estrogen receptors, the glucocorticoid receptors and the pregnane X receptors (PXR). The genetic variations of these receptors have not been well studied. (9 Currently, the only pharmacological speculation that we can use to explain these unusual patients needing very high doses of psychiatric drugs to get therapeutic concentrations is that they have unusual genetic profiles at the nuclear receptor levels, which currently cannot be tested. It cannot be ruled out that advances in pharmacology may lead to alternative hypotheses.
We hope that these 2 unusual patients will raise the awareness of epileptologists and psychiatrists that some patients may be very sensitive to the inductive effects of carbamazepine and can be identified by the need for very high doses of carbamazepine to get therapeutic concentrations. No similar cases have been well described in the past, which is not surprising since the literature in neurology82 and psychiatry83 on inducers is systematically biased, trying to deny the clinical relevance of inducers.














