Introduction
Music is the language of emotions. It is present in all cultures and has accompanied human kind since the dawn of history. It is estimated that the human species has known music for approximately 200,000 years,HYPERLINK \l "mkp_ref_001" a period sufficient to foster the generation of specific brain modules for processing musical elements such as melodic contour, tonal intervals, timbre, musical syntax, rhythm and emotional expression. (HYPERLINK \l "mkp_ref_002"
In recent years, there have been multiple investigations of brain areas that process musical emotions. For example, highly pleasing music, which generates autonomic activation states referred to as "chills" or "musical frisson", correlates with the release of dopamine in the ventral striatum, (HYPERLINK \l "mkp_ref_003" a fundamental mesolimbic structure for reward experiences. Other studies show that in contrast to non-harmonic music, harmonic music generates positive emotional responses by activating bilateral frontomedial structures, the pre-cuneus and occipital regions, the latter for visual-auditory integration. (HYPERLINK \l "mkp_ref_004" Similarly, the activation of the left anterior insula has been demonstrated to be of substantial relevance when a subject is exposed to music with consonant harmonic intervals (i.e., soft, stable, pleasant) (HYPERLINK \l "mkp_ref_005" compared to subjects exposed to dissonant harmonic intervals (i.e., violent, dynamic, unstable), whose activation patterns predominate in structures such as the right parahippocampal gyrus and the right precuneus. (HYPERLINK \l "mkp_ref_006" In fact, the comforting and pleasurable experience of music can be lost after brain injury, such as strokes or tumors, which disconnect right temporal structures from the nucleus accumbens, causing musical anhedonia. (HYPERLINK \l "mkp_ref_007"
Taken together the neuroscience research has shown that many frontal and temporal structures are involved in the emotional processing of music.
Frontotemporal dementia (FTD) is an umbrella clinical term that encompasses a group of neurodegenerative diseases characterized by progressive deficits in behavior, executive function or language. (HYPERLINK \l "mkp_ref_008" The behavioral variant (bvFTD) is the most common clinical syndrome of FTD and accounts for nearly 60% of cases. (HYPERLINK \l "mkp_ref_009" The early stage is typically characterized by changes in personality and behavior, with signs of disinhibition, loss of personal and social awareness and lack of insight into the present condition. (HYPERLINK \l "mkp_ref_010" Neuroanatomical studies have shown that bvFTD is associated with different subtypes of atrophy in limbic and paralimbic areas, includ ing loss of cerebral cortex restricted to the frontal lobes (frontal dominant); anterior frontal and temporal regions atrophy (frontotemporal subtype); intermediate and lower, predominantly right, temporal regions atrophy (dominant temporal) and loss of volume in medial frontal regions with extension to temporoparietal structures (temporofrontoparietal subtype). (HYPERLINK \l "mkp_ref_011" It is important to mention that in all the anatomical subtypes of bvFTD, various degrees of damage are involved to structures such as the anterior cingulate, the amygdala and the insula, which are fundamental for social cognition, empathy and recognition of emotions in others. (HYPERLINK \l "mkp_ref_012" Several investigations have revealed that subjects with bvFDT have difficulty recognizing emotional signals from multiple processing modalities. For example, the recognition of positive, negative and self-conscious facial expressions is significantly altered in the bvFTD patient compared with healthy subjects and/or individuals with Alzheimer's disease. (HYPERLINK \l "mkp_ref_013" Similarly, the recognition of vocal emotions (i.e., emotional prosody) is altered in the bvFTD subject, an aspect that, together with faults in the recognition of facial emotions, contributes to the abnormal social behavior exhibited by these patients. (HYPERLINK \l "mkp_ref_014",HYPERLINK \l "mkp_ref_016"
Few studies have evaluated the recognition of musical emotions in bvFTD patients. In one study, 11 patients with semantic dementia (SD), 12 with Alzheimer's disease and 20 healthy control participants underwent tests of emotion recognition in 2 modalities: unfamiliar musical tunes and unknown faces. Patients with SD were most impaired with the recognition of facial and musical emotions, particularly for negative emotions. (HYPERLINK \l "mkp_ref_017" Another study investigated the neuroanatomical substrate for recognition of musical emotion in a cohort of 26 patients with FTD (16 with bvFTD and 10 with SD). Patients with FTD showed deficient recognition of canonical emotions (happiness, sadness, anger and fear) from music as well as faces and voices compared with healthy control subjects. (HYPERLINK \l "mkp_ref_018" Other studies that have focused on differences in pitch and melody discrimination, detection of pitch errors in familiar melodies, and naming of familiar melodies in FTD, SD and Alzheimer disease, showed that patients with SD had considerable difficulty naming familiar melodies and also scored the lowest when asked to identify pitch errors in the same melodies. (HYPERLINK \l "mkp_ref_019"
The objective of this research is to assess the ability of bvFTD patients to recognize musical stimuli with emotions, such as happiness, sadness, fear and peacefulness, compared to healthy subjects, using non-familiar piano pieces. Due to the alterations in social cognition and particularly the lack of empathy, we hypothesize that we will find worse performance in the recognition of music emotions in the group of considered cases.
Material and methods
Participants
A group of 12 patients with bvFTD was selected. The subjects were recruited at the Memory Clinic of the La Inmaculada Clinic and in the private practice of the researchers in Bogotá, Colombia. Patients between 50 and 72 years of age were included, with a minimum of 3 years formal education, with adequate hearing and comprehension capacity, who fulfilled criteria for possible major frontotemporal neurocognitive disorder behavioral variant according to DSM 5, (HYPERLINK \l "mkp_ref_020" in mild or moderate stages. All patients underwent a complete neurological examination. Magnetic resonance brain imaging was reviewed, finding typical patterns of mild to moderate atrophy in frontotemporal structures in all cases. No genetic tests were done. We excluded patients with a history of moderate or severe cranioencephalic trauma in the last 10 years, a history of cerebrovascular disease and/or those who presented psychotic symptoms or delirium during the neuropsychiatric interview.
The control group consisted of 24 subjects, paired 2:1 by age and schooling. The criterion of normality was based on adequate cognitive performance in screening tests (see below).
The control subjects signed an informed consent form. Patients with bvFTD, along with a family member, also signed the informed consent. The ethics committee of the institution approved the study. No musicians were recruited as participants.
Sample size calculation
Several considerations were used to calculate the sample size: a power of 80%, an a error of 0.05% and a confidence interval of 95%. Because we adopted a paired case-control study, the sample size was initially calculated for the continuous variables outcome type based on the formula: n=f (α,B)S2/Δ21. Subsequently, the sample size was corrected by adjusting it to a ratio of 2 controls per case. The calculation established a minimum sample size of 11 cases and 22 controls for a total of 33 subjects as a minimum. This calculation was increased by 10% as a population correction factor. Thus, 36 subjects were determined as the final sample size for the study.
Cognitive screening tests
Two cognitive screening tests were applied: the Montreal Cognitive Assessment (MoCA) (HYPERLINK \l "mkp_ref_021" and INECO Frontal Screening IFS). (HYPERLINK \l "mkp_ref_022"
The MoCA is designed for the early detection of neurocognitive disorders. The assessment measures performance in the following domains: attention, concentration, executive functions (including the capacity for abstraction), memory, language, visuoconstructive abilities, calculation and orientation. The required administration time is approximately 10 minutes. The maximum score is 30, and a score equal to or greater than 26 is considered normal. (HYPERLINK \l "mkp_ref_021"
IFS is a tool designed to evaluate different cognitive domains in disorders that affect frontal functioning. In this test, 3 groups of cognitive tasks are presented: a) inhibition of responses and change of sets; b) abstraction capacity, and c) working memory. IFS is a useful means to differentiate bvFTD from other dementia types, such as Alzheimer's disease. (HYPERLINK \l "mkp_ref_023" The IFS cut-off point suggested for the Colombian population is 17.5 (sensitivity: 92.8%; specificity: 86.3 points). (HYPERLINK \l "mkp_ref_024"
Test of musical emotion recognition
We considered 56 musical fragments that have been experimentally validated in Western cultureHYPERLINK \l "mkp_ref_025" and represent four emotional categories: happiness, sadness, fear and peacefulness. The fragments were grouped into sets of 14 musical stimuli for each emotional category, with a duration per fragment that ranged from 9.2 to 16.4 seconds.
The theoretical paradigm of this test is based on considering music as an auditory stimulus capable of evoking emotions that can be easily recognized and labeled by any subject with or without musical training. For example, the fragments that denote happiness are written in the major mode with a fast tempo and medium-high melodic lines. The musical fragments for sadness adopt a slow tempo and the minor mode. The scary excerpts consist of minor chords in the third and sixth degrees of the scale, implying the use of notes outside the initially established key. In addition, the chords can be irregular and with dissonant intervals. Finally, the musical fragments that express peacefulness are characterized by an intermediate tempo, the major mode and interpretation based on arpeggios. Each of the 56 fragments (i.e., tracks) was digitally generated and had the quality of a piano tone. (HYPERLINK \l "mkp_ref_025"
Process
To control environmental noise, the evaluation was performed in a closed area. The music fragments were reproduced in high quality using a Sony personal audio system model SRS-X3 at 50-60 dB. Each subject received a sheet on which were written the four emotions to be evaluated. Subsequently, the testing method was explained using 2 musical examples, one from the soundtrack of the film "Jaws" and the other from the soundtrack of the film "Schindler's List", which represented fear and sadness, respectively. Once the participants understood the test, the 56 musical tracks were randomly reproduced, with an interval of 5 s between each track. Each response was recorded by the evaluator. Each track was played only once.
Statistical analysis
The collected information was analyzed using the statistical software SPSS released in 2007 for Windows, version 16.0, Chicago. The Kolmogorov-Smirnov normality test was performed to evaluate the distribution of the variables under study. The test found a normal distribution of the majority (K-S, P > .05), with the exception of MoCA (P = .06) with non-normal distribution. Thus, non-parametric statistics tests were applied to this variable. The descriptive statistics of the quantitative variables were established by measuring central tendency and dispersion (i.e., mean, median, standard deviation, and interquartile range). For the categorical variables, frequencies were described.
In order to ensure balanced groups and maintain between-group comparability, assessing baseline was performed, taking care that demographic variables, such as age, gender and years of schooling, did not display statistically significant differences between the two groups (unpaired samples t-test: P>.05).
To evaluate the occurrence of statistically significant differences in the recognition of musical emotions between the two groups, hypothesis tests of parametric statistics (a two-way Anova) were performed comparing the (average) measurement of successes in the recognition of each musical emotion and the total test scores in each group. Additionally, the relationship between musical emotion recognition performance and the neuropsychological screening scale scores (MoCA) was evaluated using dispersion graphs and nonparametric linear regression.
Results
Demographic information
The patients with bvFTD included in the study (n=12) presented a mean age of 64.6 ± 6.3 years, a male gender proportion of 58.3% (n =7) and an average of 11.5 ± 3.87 years of education. The control group (n=24) had similar charac teristics, maintaining between-group comparability (P>.05) ( HYPERLINK \l "t1" ).
Neuropsychological screening
For the control group, the subjects obtained a MoCA score median of 27.5 [interquartile range, 6]. For bvFTD group, the MoCA score was 14.5 [17]. For IFS, the control group obtained a score of 24 [3.3], while subjects with bvFTD scored 8.6 [6.4]. As expected, statistically significant differences were found between the 2 groups (P < .001) ( HYPERLINK \l "t1" ).
Recognition of musical emotions
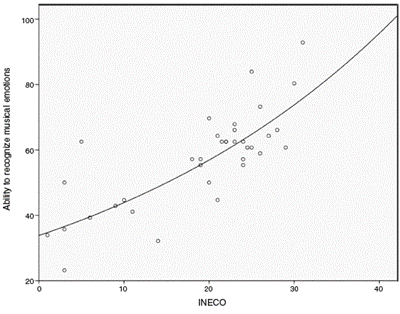
In patients with bvFTD, the total mean of correct answers on the test was 23.6 (42.26%). In contrast, for the control sub jects, the average number of correct answers was 36.3 (64.8%). The ability to recognize musical emotions was significantly lower in bvFTD patients compared to controls; statistically significant differences were found for each of the evaluated musical emotions and in the overall test (F(134) = 40,71; P < .001) (figure 1).
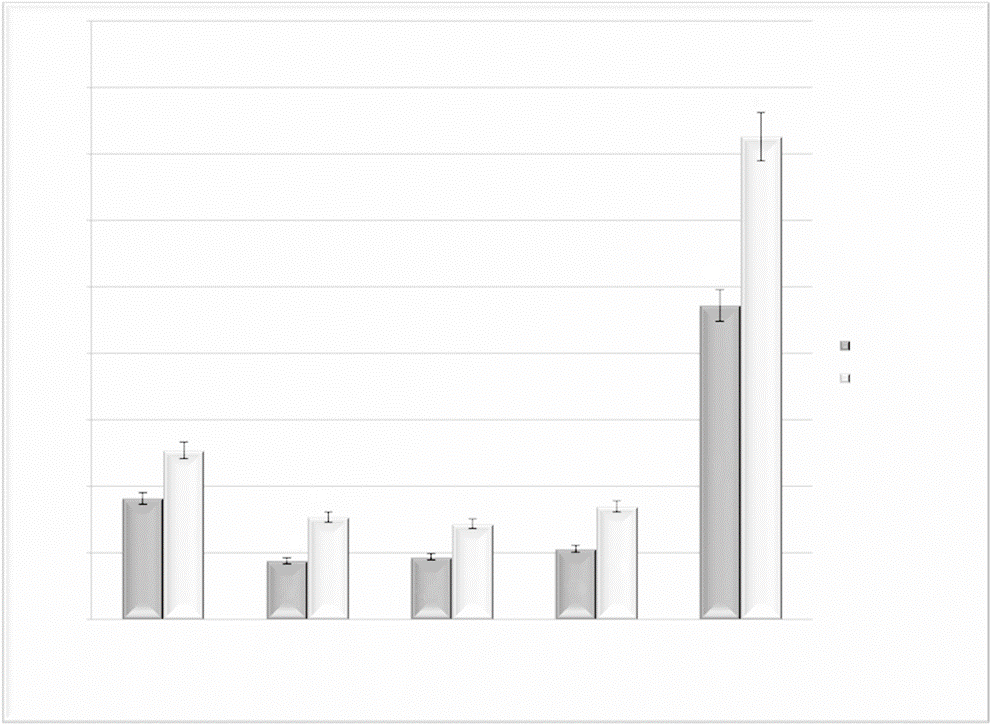
Figure 1 Recognition of musical emotions in patients with bvFTD and control subjects. Statistically significant differences were found for each of the musical emotions evaluated and in the overall test, with the controls being those with the best performance. SD: standard deviation.
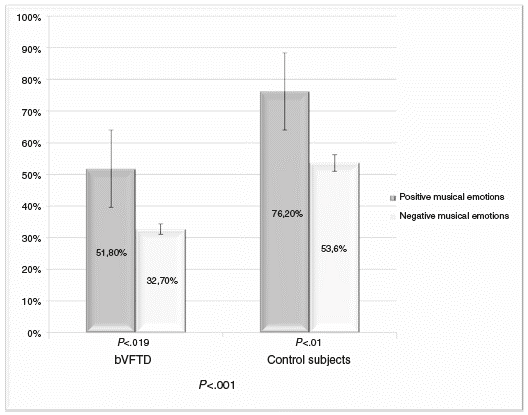
Figure 2 Comparison of the ability to recognize positive and negative musical emotions in patients with bvFTD and control subjects. Statistically significant differences were found in both groups in recognizing negative musical emotions, compared with positive musical emotions, with patients with bvFTD having the worst performance.
In addition, the ability to recognize positive (happy, peace ful) and negative (sad, scary) musical emotions was evaluated. The control group exhibited a statistically significant better performance in the ability to recognize positive and negative musical emotions compared to the bvFTD group (F(134) = 29,6; P < .001). In the same way, patients with bvFTD had less percentage of correct answers in the recognition of negative emotions compared with positive emotions (F(134) =15,3; P<.001) (figure 2).
The control group also had greater difficulties recognizing negative musical emotions compared with positive emotions. The within-groups statistical test revealed important differences in the recognition of positive versus negative musical emotions, which represents a statistically significant difference regarding the recognition of positive compared to negative emotions in both groups.
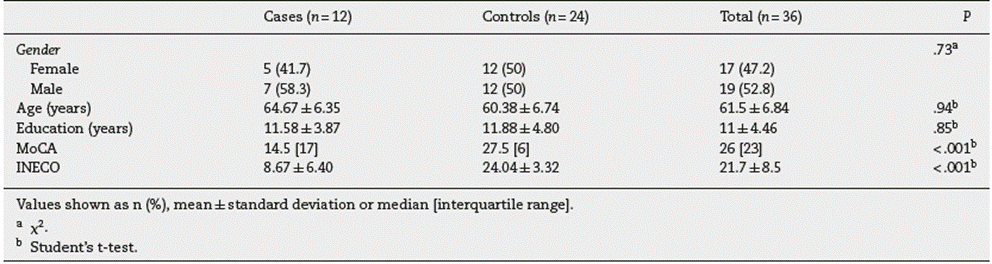
The relationship between the recognition of musical emo tions and the MoCA score was evaluated. We observed a trend of simultaneous increase in the 2 variables. However, a strong linear association between the 2 variables was not found (r = .74). Only 55.1% (r2 = .55) of the change in the proportion of total successfully recognized musical emotions was explained by the degree of cognitive deterioration detected with the MoCA (F = 51.45; P < .001) (figure 3).
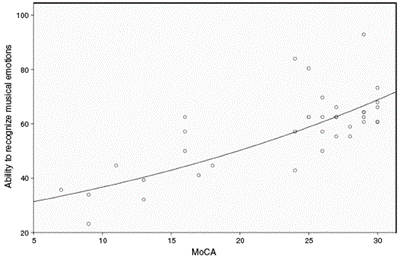
Figure 3 Relationship between the recognition of musical emotions and the MoCA score. The total of the sample showed a relationship of simultaneous increase in the MoCA test and the ability to recognize the general musical emotions (r = .74; r2 = .55; F = 51.45; P< .001).
Similar results were found when performing this same analysis with IFS, whereby only 60% of the changes detected in the bvFTD group by this instrument were explained (r=.77; r 2 = .602; F = 51.48; P < .001) (figure 4).
Discussion
This study investigated the recognition of musical emotions in subjects who have suffered damage to frontotemporal structures in the context of a neurodegenerative disease. The most relevant results were that patients with bvFTD had greater dif ficulties, compared with healthy subjects, in the recognition of musical fragments that transmitted emotions, such as happi ness and peacefulness, and had even more difficulties in those with sadness and fear sounds (figure 1).
Our study revealed that both, healthy and bvFTD subjects, found it easier to recognize positive than negative musical emotions (figure 2). That is, the general sample was more sensitive in identifying musical pieces that conveyed happiness and peacefulness than sadness and fear. The within-group analysis showed the difficulty experienced by both groups in recognizing negative musical emotions, whereby the subjects with bvFTD performed worse. This finding is impor tant because many patients with bvFTD exhibit aggressive, impulsive and become less sensitive to others feelings and needs, symptoms that reveal lack of empathy. Poor recognition of negative emotions such as fear and sadness, which often signal distress and need to others, may be interpreted as a lack of empathy and concern in the patients. Consistent with this, caregivers often describe patients with bvFTD as showing a lack of emotional concern for others. (HYPERLINK \l "mkp_ref_013" In the case of control subjects, the facility to recognize positive musical emotions, particularly the happiness, is consistent with other studies in a healthy population. (HYPERLINK \l "mkp_ref_025"
Our results are also consistent with those of other studies that find the recognition of social emotions is compro mised by FTD. Such studies provide ample evidence regarding the difficulty faced by these patients in recognizing facial emotions, (HYPERLINK \l "mkp_ref_026"-HYPERLINK \l "mkp_ref_028" in adapting to alterations in the processes of cognition and affective, as well as moral empathy, (HYPERLINK \l "mkp_ref_015",HYPERLINK \l "mkp_ref_029" in coping with defects in the theory of the mind, (HYPERLINK \l "mkp_ref_030",HYPERLINK \l "mkp_ref_031" and in rec ognizing emotional prosody. (HYPERLINK \l "mkp_ref_032"-HYPERLINK \l "mkp_ref_034"
Musical emotions belong to this spectrum of social emo tions, since music is a non-verbal language that facilitates cohesion and emotional synchronization in human groups. Some studies, previously mentioned in the introduction, sup port our results in relation to the flawed recognition of musical emotions in FTD patients and link it to insular and amygdala atrophy as well as volume reduction of the right temporal pole and the anterior inferior regions of the left temporal lobe. (HYPERLINK \l "mkp_ref_017" Of substantial importance is dysfunction of the amygdala in the bvFTD patients, which is clinically manifested by disinhibition, hyperorality and hypersexuality (i.e., Klüver-Bucy-like symptoms). (HYPERLINK \l "mkp_ref_035" This structure is fundamental for the process ing of emotional and socially relevant information, including threatening stimuli. Several studies have demonstrated, in dif ferent clinical contexts, that amygdala damage prevents the recognition of sad or fearful music, (HYPERLINK \l "mkp_ref_036" as documented by our patients with bvFTD.
Regarding the link between theory of mind, social cog nition and musical emotions, musical recognition could be considered a form of mental attribution that is performed on an abstract stimulus, in this case, music. It has been found that emotional musical recognition in bvFTD patients is associated with the activation of an extensive bilateral neu ral network (maintained in part by von Economo neurons), which include the insula, the anterior cingulate, the prefrontal cortex, the orbitofrontal cortex, the prefrontal medial and dor sal prefrontal cortex, the anterosuperior temporal cortex, the fusiform gyrus and the parahippocampal gyrus. Similarly, limbic areas, such as the amygdala and the hypothalamus, are involved, as well as subcortical regions, including the nucleus accumbens and the ventral tegmental area. (HYPERLINK \l "mkp_ref_018"
Finally, in our study, we analyzed the proportion of correct answers in relation to the MoCA and IFS cognitive screen ing tests (figure 3 and figure 4). We found that the poorer the cognitive performance of the subjects was, the worse their ability to recognize musical emotions. In IFS, which evaluates frontal/executive functions with greater precision than the MoCA, 60% of correct answers were explained by the degree of cognitive deterioration. This outcome indicates that additional variables are probably involved in the recogni tion of musical emotion, for example, the subject's profession (i.e., musician versus non-musician), the degree of fondness for and curiosity regarding music, the lyrical versus melodic component of the music, the cultural aspects of musical recognition, and medical comorbidities (e.g., temporal lobe epilepsy). In our study, no musicians were recruited as participants.
One limitation of the study was the difficulty that subjects experienced in differentiating sad from peaceful music, probably because sad music typically generates a relaxing effect. The control subjects also exhibited sub-optimal performance in this aspect of the test. They achieved a total score of 64.8% correct in identifying the music fragments in our study, while in other studies, they achieved scores higher than 80% HYPERLINK \l "mkp_ref_025",HYPERLINK \l "mkp_ref_036". In future research, we consider important to separate bvFTD patients according to the anatomical subtypes described in the introduction (i.e., frontal dominant, frontotemporal, tempo ral dominant and temporofrontoparietal) and to correlate the structural and functional changes of the neural networks com promised by the disease with the ability to recognize musical emotions.