Introduction
In Colombia, plenty of factors that affect patients, such as long distances, extended times for authorization of remissions and the lack of higher complexity referral centers, increase the need of technological tools for the first level of medical care to facilitate and support remote medical treatment through real-time communication with specialized staff, and monitoring of physiological and electrocardiographic changes that allow for an immediate remote compatibility with medical specialties. In order to create technological tools to address this issue, domestic companies must produce the necessary hardware and software for adjusting medical devices to the needs of the country and implementing real-time communication technologies to create a real continuous care network to treat a referred patient.
Vital signs monitors (VSM), also known as physiological parameter monitors, are electronic devices that measure and display biological information about patients under constant monitoring. They are often used with patients suffering from critical illnesses, where heart rate, electrocardiographic signal, arterial pressure and oxygen parameters, as well as temperature measurements provide essential diagnostic information that enables the medical staff to make decisions on the treatment to be used in each pathology 1,2.
Because of the importance of VSM in current health care services, it is necessary to perform a process of clinical validation of these devices to guarantee enough reliability and accuracy before establishing their daily use in a clinical context. Taking this into account, this study describes the clinical trials of the SignCare VSM, manufactured by Fundación Cardiovascular de Colombia (FCV), by means of comparing its measurements with a commercial device (Mindray PM-8000) and with the manual method of vital signs measurement, as suggested by Association for the Advancement of Medical Instrumentation (AAMI) 3,4.
Materials and procedures
SignCare VSM description
The SignCare monitor is an integrated system that allows recording physiological parameters such as heart rate, breathing, temperature, oxygen saturation and NIBP, and then displays the information on the integrated monitor screen. Additional equipment has data transmission systems that send physiological parameters data via Internet, allowing the use of information in real time through a web platform controlled by a central monitoring station.
The monitor includes rechargeable batteries that provide energy independence for at least three hours; additional equipment includes an adapter that allows charging the battery, thus ensuring continuous use. Portability features involve a user-centered design, and an easy and intuitive touch screen for handling. The hardware is FPGA-based (field programmable gate array) and uses an FDA-Approved
Real-Time Operative System (uC-OSII). This device was validated in a laboratory in order to ensure a robust prototype, with the same level of quality as commercial monitors 3-5.
This device represents a tool for health care and communication improvement in the processes involving the referral of patients to higher complexity health centers, giving place to opportunities for future developments and applications according to the different needs of the country. The continuous monitoring and communication with the receiving health centers while they await for the patients will represent better life expectancies, particularly in cardiovascular, gynecological, obstetrical, respiratory, pediatric and trauma services, by speeding up the whole process and increasing control of the risk factors of patients.
Design of the study
A cross-sectional study was performed, following a validated protocol based on the recommendations by AAMI for monitoring equipment validation 3-5. The study protocol consisted of the following items:
Clinical assessment of the SignCare VSM
Various tests were performed to compare heart rate, breathing, oxygen saturation, temperature, NIBP measurements and the waveforms obtained through the SignCare VSM, against those obtained with Mindray PM-8000 VSM. The patients in this study were selected through a non-random sample of the population that attended the emergency service of the FCV. The sample size was calculated using Epidat 3.1, with a 95%CI, a base population from the metropolitan area of 1 000 000 approx., and a design effect of 1 6,7. Based on the previous calculation, 98 subjects were included in the study, a sample size with sufficient statistical capacity to test differences in each of the clinical variables measured by both monitors (Table 1). Individuals with background of cardiovascular, mental or sensorial perception diseases, that could compromise the understanding of the study procedures, as well as pregnant, neonate and pediatric patients, were excluded.
Table 1 Sample calculation for the clinical trial.
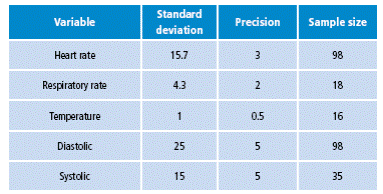
Source: Own elaboration based on the data obtained in the study.
The subjects were chosen during their stay in the emergency service of FCV, where tests were performed in a temperature-controlled room (20°C), in decubitus supine position.
Personal data variables of all participants such as age, sex, origin and comorbidities were taken into account. Each patient was monitored for approximately 30 minutes, obtaining three simultaneous measurements of physiological parameters through SignCare VSM and Mindray PM-8000 VSM. Two trained nursing staff members did the qualitative assessment of the morphology of the registered electrocardiography waves, providing visual evidence for each patient. For all physiological parameters, statistical evidence was used in order to establish the correlation degrees between both monitors.
Statistical analysis
The vital signs measurements collected from 98 patients using the SignCare VSM and a commercial monitor were analyzed. A general description of the variables of interest was done; for this purpose, continuous variables were described as averages with standard deviation (SD) and the variables category as absolute (n) and relative (%) frequencies. Also, the comparison of measurements obtained with the SignCare VSM and the commercial Mindray PM-8000 VSM was performed.
This study suggests a statistical hypothesis of equality between the results of both monitors to prove the effectiveness of the SignCare VSM, while having the commercial monitor as a reference. Comparisons with a p value lower than 0.05 were considered as statistically significant differences. Furthermore, Pearson correlation coefficients between measurements of both monitors were established according to variable distribution.
The data were transferred to digital files using Microsoft Office Excel. The database was cleaned and analyzed using Stata 12.1 (Stata Corporation) for results.
Ethical considerations
This study was approved by the research ethics committee of FCV, under the principles established on the Helsinki Declaration and on the Resolution 8430 of 1993 by the Ministry of Health of Colombia. Since this study is classified as non-invasive, it constitutes a low risk research as stipulated by article 10 of the aforementioned resolution; therefore, there was no risk to the health of the subjects that took part in it. All participants entered the study after signing an informed consent where risks, benefits and confidentiality of information were expressed.
Results
Clinical assessment of the SignCare VSM
The average age of the 98 subjects was 51.08±19.75 and 50% of the patients were female. 89.8% did not declare any comorbidity, and the remaining 10.2% were divided into 10 types of comorbidities.
Some of the comorbidities registered were atrioventricular block (1.02%) and aortic valve dissection (1.02%). In the clinical study, three samples per patient were taken for each physiological parameter with both the SignCare VSM and the commercial VSM. When comparing the vital signs measurements obtained by both monitors, there were not statistically significant differences for most of them.
Statistically significant differences were only found for the following variables: respiratory rate (resp/min) in sample 1 (p=0.002); respiratory rate (resp/minute) in sample 3 (p=0.000); body temperature (°C) in samples 1 (p=0.000) and 3 (p=0.038), and systolic arterial pressure (mmHg) in sample 1 (p=0.046). However, these differences were not clinically relevant and do not represent a difference that may affect the patients' health since the difference margin is minimum. The main results are shown in Tables 2 and 3.
Table 2 Description of the general characteristics of the patients studied.
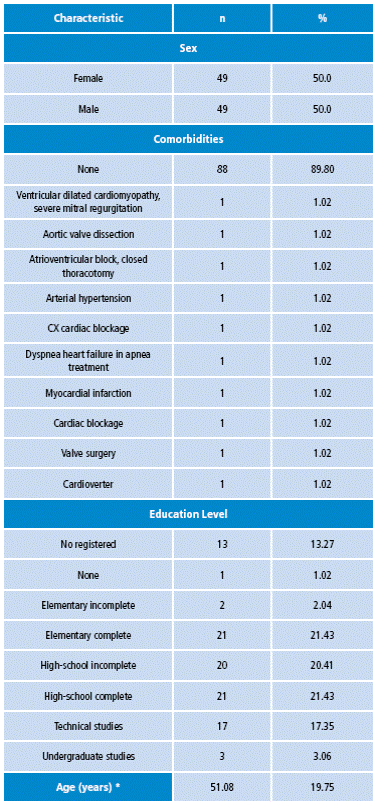
* Average and standard deviation of age in years. Source: Own elaboration based on the data obtained in the study.
Table 3 Comparison of measurements obtained with the SignCare Vital Signs Monitor and the commercial monitor.
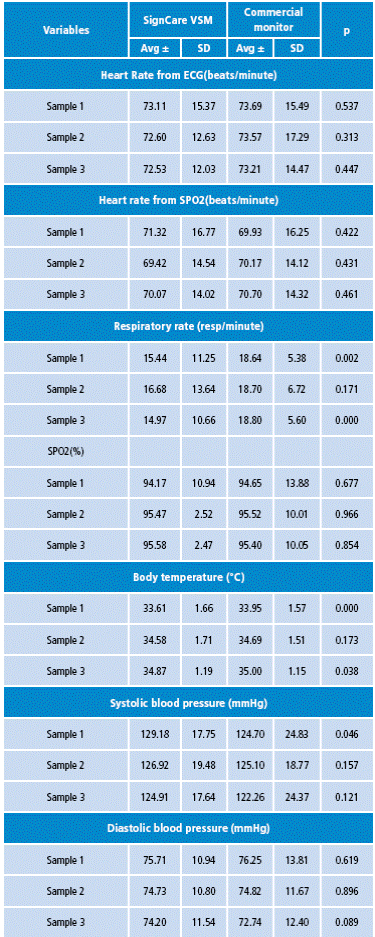
SD: Standard deviation; p: p value determined by Student's t-test. Source: Own elaboration based on the data obtained in the study.
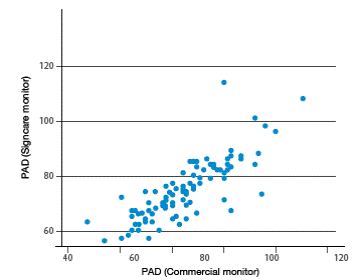
After analyzing all measurements, high correlation levels among vital signs measured by both monitors can be observed. Higher correlations were found for heart rate obtained from ECG (r=0.844), heart rate obtained from SPO2 (r=0.821), body temperature (r=0.895), systolic blood pressure (r=0.780) and diastolic blood pressure (0.811). Moderate correlations were found for respiratory rate (r=0.498) and SPO2 (r=0.603). Figures 1-4 show correlation between both devices.
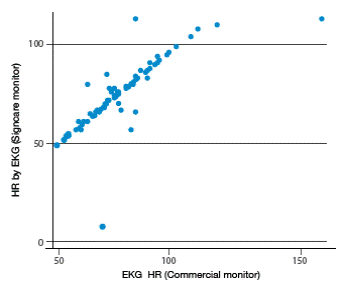
Figure 1 Correlation of heart rate measurements obtained from ECG between the two vital signs monitors. Source: Own elaboration based on the data
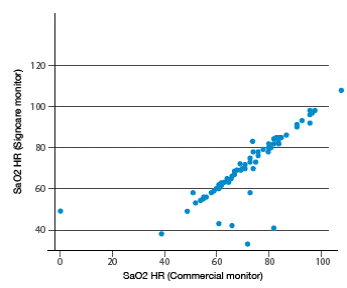
Figure 2 Correlation of heart rate measurements obtained from SPO2 between the two vital signs monitors. Source: Own elaboration based on the data obtained in the study.
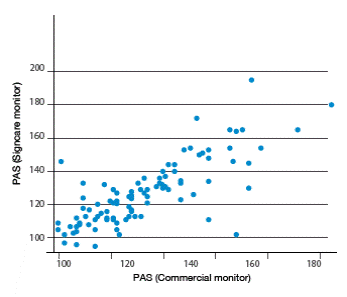
Figure 3 Comparison of systolic blood pressure between the two vital signs monitors. Source: Own elaboration based on the data obtained in the study.
Discussion
This paper shows that the SignCare VSM reached high degrees of correlation with vital signs values for heart rate, SPO2, temperature, NIBP, and respiratory frequency obtained through the Mindray PM-8000 device. The analysis conducted using the Student's t-test allowed confirming that the SignCare VSM is as reliable as the Mindray PM-8000 VSM for the qualitative detection of morphologic alterations in electrocardiographic records and the physiological parameters of breathing, temperature, oxygen saturation and NIBP.
Although some samples of respiratory rate, body temperature and systolic blood pressure had a low statistical level of correlation, it does not represent a clinically significant margin, since differences are minimal. Doing a quantitative assessment of the electrocardiographic parameters was not possible since these devices cannot print nor store biological signals. Because of this, two trained evaluators, using photos and videos, registered in a form the presence or absence of alterations in the morphology of the p wave, QRS complex, ST segment and T wave in electrocardiography measurements of both monitors. In conclusion, it is considered that the SignCare VSM fulfilled the validation criteria issued by AAMI, which makes possible its recommendation for clinical use in adult population.