Introduction
Gangrene means cell necrosis 1 and may be caused by various microorganisms: Pseudomonas aeruginosa, Estaphylococcus aureus, Streptococcus pyogenes2, Clostridium spp and other anaerobic bacteria 3,4. When gas is present and evolution is fast, gangrene is caused by bacteria of the genus clostridium, whose spectrum of infection includes contamination and anaerobic cellulite before myonecrosis 5.
Clostridial myonecrosis or gas gangrene is very rare in pediatric population; its origin can be traumatic 6 due to the continuity to the inoculum, or spontaneous due to hematogenous spread from the gastrointestinal tract. The first is mainly caused by Clostridium perfringens7 and the second usually by Clostridium septicum8.
C.septicum is a Gram positive anaerobic bacillus that forms spores, found in 2% of healthy population at the cecum and the ileocecal area, where poor vascularization, pH and osmotic and electrolytic characteristics -associated with lymphocyte protection imbalance- 9 favor their proliferation 10. The microorganism has tolerance relative to oxygen, which facilitates its proliferation in healthy tissues, so that the inoculum required for infection is 300 times smaller than that for C. perfringens11.
The proliferation of Clostridium produces large amounts of hydrogen and carbon dioxide; these gases are distributed in tissue planes, are dissected and generate palpable emphysema. The necrotic process extends to adjacent healthy tissue, causing massive necrotizing gangrene within hours 12.
The rapid proliferation and systemic toxicity generated by C. septicum is considered to be caused due to the production of four exotoxins -alpha toxin, cytolysin, β-toxin and neuraminidase-13; alpha toxin 14, the most pathogenic of all, is responsible for intravascular hemolysis, tissue necrosis and increased capillary permeability -pore-forming cytolysin- 15. Other functions of this toxin include sphingomyelinase activity, favoring the increase of platelet aggregation, reducing the adhesion of polymorphonuclear 16 and suppressing muscle contraction 17. Tissue destruction results in edema and ischemia, which consequently cause severe metabolic acidosis, fever, DIC and renal failure secondary to the effects of hypotension, myoglobinuria and direct toxin nephrotoxicity 10. Genomic studies of the bacterium show highly conserved nuclear sequences 18.
Case presentation
17 year old teenager with a history of Rosai-Dorfman histiocytosis, autoimmune lymphoproliferative syndrome, decreased T helper lymphocytes, with two episodes of febrile neutropenia, recurrent stomatitis and two episodes of pneumonia. The patient attended the emergency room due to a clinical picture of pain for 16 hours in the anterolateral side of the left leg, with limited mobility and gait; acetaminophen and optimal analgesia was administered for management of fever and pain at home. Two hours before consultation, a rapidly progressive edema had begun in the same location of the lower left limb. A diarrheal episode occurred a week before, which resolved spontaneously, and no traumas were referred.
On admission to the emergency room, the respiratory rate was 23 breaths/min, the heart rate of 112 beats/min, body temperature of 38°C and blood pressure of 105/74mmHg; the left inguinal region presented erythema, limbs with edema, cyanosis and erythema, crackles in the left lower limb in its entirety, capillary filling greater than three seconds, difficult palpation pulse and no other abnormalities on physical examination. Gas gangrene was considered and antibiotic treatment was administered with vancomycin and meropenem, as well as analgesia with morphine.
X-rays of the abdomen and left lower limbs showed subcutaneous emphysema in the thigh, leg and pelvis (Figure 1); a deep vein thrombosis with signs of gangrene and compartment syndrome was determined, so a fasciotomy was conducted as a vital urgency.
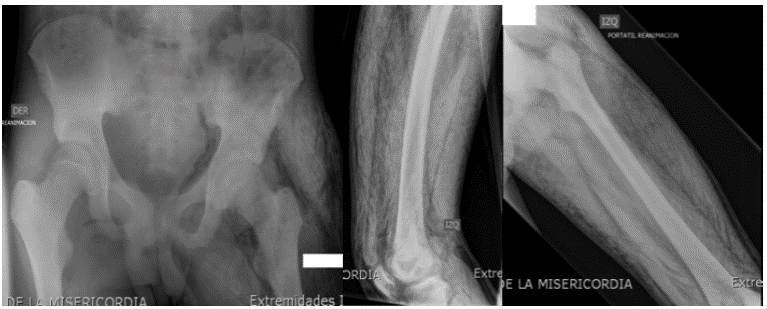
Figure 1 Radiographs of the left thigh in lateral projections, AP and hip, where the presence of gas dissecting the muscle planes and subcutaneous tissue is visible, with no evidence of bone involvement. Source: Own elaboration based on the data obtained in the study by the Radiology Service of Fundación Hospital de la Misericordia in Bogotá.
In the operating room, increased mottling purple coloring and extension to the lower abdomen and right thigh was observed, along with blebs of serohematic not fetid content in the inguinal region and presence of marked edema in the left scrotal sac (Figure 2); after examining the subcutaneous tissue and fascia, abundant gas production, thrombosed venous vessels of small caliber, marked tension in the lateral compartment of the left thigh with full pressure in the vastus lateralis when cutting the fasciae latae were found.
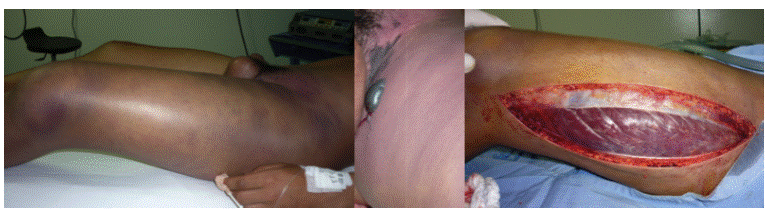
Figure 2 Pictures taken in the operating room previous and during fasciotomy, where purplish skin coloration, marked edema, blisters in the inner side of thigh and vastus lateralis pressure are observed. Source: Own elaboration based on the data obtained in the study by the Plastic Surgery Service of Fundación Hospital de la Misericordia in Bogotá.
By the end of the procedure, persistence of distal coldness and paleness of the left lower limb were observed; when removing surgical fields, violet color progression on the chest and ecchymosis in the left iliac fossa were found. Biopsies were taken to histopathology and penicillin and metronidazole (Figure 3) were administered. Laboratory results are shown in Table 1.
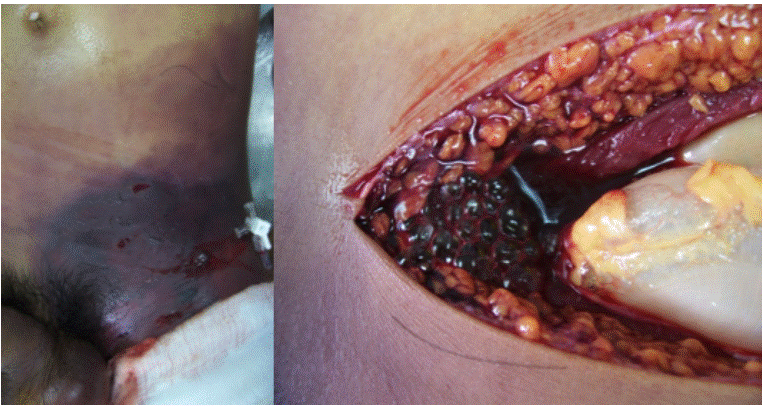
Figure 3 Postmortem picture where accentuation of violet color in the skin and scrotal edema are observed. Aspect after cutting the muscle and tissues where the expelling of bubbles caused by the gas that dissects the tissue are observed. Source: Own elaboration based on the data obtained in the study by the Pathology Service of Fundación Hospital de la Misericordia in Bogotá
Table 1 Laboratory exams
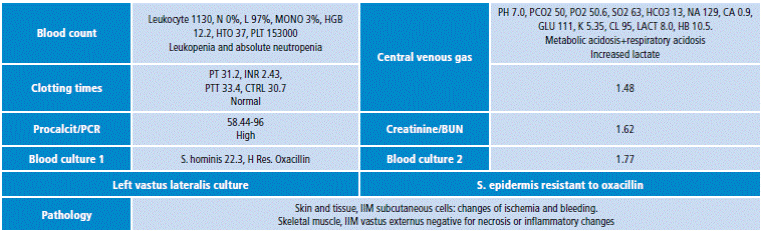
Source: Own elaboration based on data taken from the clinical profile of the patient.
Immediately after the surgical procedure, the patient was transferred to the pediatric intensive care unit, where he was admitted hypoperfused with hemodynamic deterioration, requiring high ventilatory parameters and with diastolic hypotension; then, he suffered a cardiac arrest with asystole unresponsive to resuscitation and died.
Discussion
In recent decades, most cases of gas gangrene in adults have been associated with intestinal malignancy 19-22, recent surgery, trauma, diabetes or peripheral vascular disease 23; between 1 000 and 3 000 cases of this disease are reported per year in the United States 24,25.
In children, a prevalence of Clostridium spp. of 7% in all isolates of anaerobic bacteria has been reported 26; there is no difference in the distribution between genders and most cases of C. septicum are associated with leukemia, immunodeficiency (as in this case), cyclic neutropenia 27, hemolytic uremic syndrome, among others 28,29. In the case of this paper, there is no clear intestinal focus, but there is a history of diarrhea a week before.
The diagnosis of clostridial myonecrosis is difficult because it may be initially confused with cellulitis, pyoderma gangrenosum 30 or necrotizing fasciitis 31; however, unlike others, the clinical course is very fast (6 to 48 hours) and there are some more specific symptoms that appear late. Thus, the presence of severe pain as the predominant symptom, as well as signs of systemic inflammatory response and gas (crepitation) should immediately lead to suspect this disease 32; progressive inflammation signs and blisters with brown liquid content appear subsequently 28.
Imaging studies like X-rays are useful since they help to identify gas in the deep tissues; similarly, computed tomography or magnetic resonance imaging can detect the spread of the infection along fascial planes.
The definitive diagnosis is made by determining the bacilli on the site of the lesion; bacteremia can be detected in up to 15% of cases and develops several hours before skin manifestations. To isolate the bacteria, special anaerobes culturing is required; the microorganism can also be found in the aspirate of the lesions or in the biopsy. Surgical findings show that the muscle does not contract with stimulus nor does it bleed, and edema and variable color are observed. Histopathology showed varying cell lysis -muscle, fascia, fat- and gas formation with absent inflammatory cells. In this particular case, the absence of necrosis signs under light microscopy can be explained by the rapid onset of the symptoms 5.
No procedure can delay or replace emergency surgical treatment and antibiotic treatment, accompanied by hemodynamic stabilization 1. Selected antibiotics include crystalline penicillin at high doses plus other antibiotics based on clinical suspicion, for example, clindamycin 33; another scheme includes administering broad spectrum antibiotics such as vancomycin, metronidazole or meropenem 34. Surgical intervention -fasciotomy, debridement, resection or amputation- determines survival since necrotic areas do not allow the arrival of the antibiotic 28.
There are no conclusive studies to recommend the use of hyperbaric oxygen in spontaneous gas gangrene; the study of immunoglobulin and granulocyte stimulating factor 9 in immunosuppressed children recently started 10 and the future of treatment leads to the inhibition of the alpha-toxin. Mortality varies between 67% and 100% 11, finding the highest values among immunodeficiency patients with underlying malignancy 35.
Gas gangrene is considered to be a fulminant disease, so initiating antibiotic therapy and surgery must not be delayed once the diagnosis is suspected. It is highly recommended to always handle these patients in pediatric intensive care units due to the high risk the disease represents.














