Introduction
Salmonella genus microorganisms are gram-negative bacilli of the Enterobacteriaceae family. The most recent classification of Salmonella includes only two species: Salmonella enterica and Salmonella bongori. S. enterica consists of six subspecies subdivided into different serotypes based on somatic antigenic factors O, flagellar H and capsular Vi. According to the Kauffmann-White scheme, recommended by the Collaborating Centre of the World Health Organization for Reference and Research on Salmonella, there are plenty of somatic and flagellar antigens, so serovars can be classified into serogroups based on the type of antiserum-O which causes agglutination in the laboratory. In this regard, Salmonella enteritidis belongs to serogroup D and corresponds to S. enterica, subspecies enterica, serovar enteritidis, a name that is ignored for practical purposes.
Salmonellosis is a global public health problem 1,2; it is an infection acquired orally and the following are considered risk factors for the development of the gastrointestinal infection in children: contact with another household member infected with the bacteria, consumption of infant formula, visits to health centers and consumption of untreated water 3. Breast milk decreases the risk of sporadic salmonellosis in infants 4,5, although there are reports that suggest that this may favor the transmission of some serovars of Salmonella that colonize the mammary glands 6.
The incubation period for the gastrointestinal infection is 6-72 hours 7 and, despite the usual self-limitation of the infection, between 1% and 5.7% of patients may develop bacteremia, which is mostly benign, although ocasional osteoarticular or meningeal secondary outbreaks 8,9 may appear. Invasive infection occurs due to the distortion of local enteric immunity and is particularly seen in the extremes of life or in individuals with predisposing conditions, particularly in immunosuppressed patients. The disseminated form is frequent in infants under two years of age and is much more common in the neonatal period, becoming an important cause of morbidity and mortality in this age group 11-14.
In developed countries, Salmonella meningitis represents less than 1% of cases of bacterial meningitis confirmed in infants and children. In contrast, the incidence reported in developing countries reaches 13% 15-21. The clinical implications of Salmonella meningitis are serious, with a mortality rate that can reach up to 89% in underdeveloped countries 22-24. In an observational study, 13% of the patients died, 75% had at least one complication during the acute phase -hidrocefalia 50%, subdural collection 42%, stroke 33%, ventriculitis 25%, empyema cerebral 13%, and brain abscess 8 %- and 71% of patients developed motor sequelae, epilepsy, language delay or cognitive delay in long-term follow-up 25. The prognosis is poor, especially, due to complications inherent to the infection, so early diagnosis is crucial to achieve a favorable outcome 26-28.
Gastrointestinal infections are usually self-limiting diseases and rarely require antibiotic treatment 29,30. A meta-analysis proved that there are no benefits in using antibiotics to treat gastrointestinal infections caused by Salmonella31; however, this is mandatory for invasive complications such as sepsis and meningitis. Broad-spectrum cephalosporins, such as cefotaxime and ceftriaxone, are preferably used in the treatment of salmonellosis in children, since fluoroquinolones are not clearly marked and the evidence for carbapenems is still insufficient 1,32.
This paper aims to present the clinical case of an infant with Salmonella enteritidis meningitis successfully treated with meropenem.
Case presentation
The patient was a two month-old infant, admitted in the pediatric emergency department of a level III hospital in Popayán, Cauca, who came from the rural area, daughter of a 31 year-old mother as a result of a second pregnancy, born by Caesarean section at 37 weeks due to hypertensive disorder during pregnancy and weight of 2 550g at birth.
At birth, the patient received BCG and hepatitis b vaccines, but was not administered rotavirus, polio, pneumococcus nor pentavalent (DPT, haemophilus influenzae type b and hepatitis b) vaccines at two months of age, which are included in the expanded immunization program for Colombia; until then, she had been fed exclusively on breast milk.
The infant had a clinical picture that started the same day of admission, consisting of a runny nose, coughing without cyanosis nor associated vomiting, fever, hyporexia and hypoactivity; she entered in the hospital in poor general condition, with 4.2kg weight, afebrile, heart rate of 145 beats/min and respiratory rate of 45 breaths/min, peripheral oxygen saturation equal to 88%, intercostal runs, pushing and staring episodes that suggested absences. No aggregated noises were found in lung auscultation and, apart from what has been described, the rest of the physical examination was normal, including fontanelar tone and consciousness. Paraclinical studies documented at admission are detailed in Table 1.
Table 1 Paraclinical results of studies on admission to the emergency room.

Source: Own elaboration based on the data obtained in the study.
Based on these findings, diagnosis of unknown origin sepsis was established; empirical antibiotic treatment with ceftriaxone was initiated at a dose of 100 mg/kg/day and urine and blood cultures 1 and 2 were ordered, as well as a lumbar puncture for cerebrospinal fluid studies (CSF). The CSF cytochemical reported hypoglycorrhachia, hyperproteinorrachia and neutrophilic pleocytosis, which suggested bacterial meningitis. The Gram staining showed moderate gram-negative bacilli and scarce gram-negative coccobacillary, confirming the diagnosis (Table 2); for this reason, suspending ceftriaxone was determined and meropenem antibiotic treatment was started at a dose of 120 mg/kg/day and the patient was admitted in the Pediatric Intensive Care Unit. Preliminary reports from blood cultures 1 and 2 confirmed the presence of concomitant gram-negative bacilli in blood.
Table 2 CSF diagnostic and control analysis.
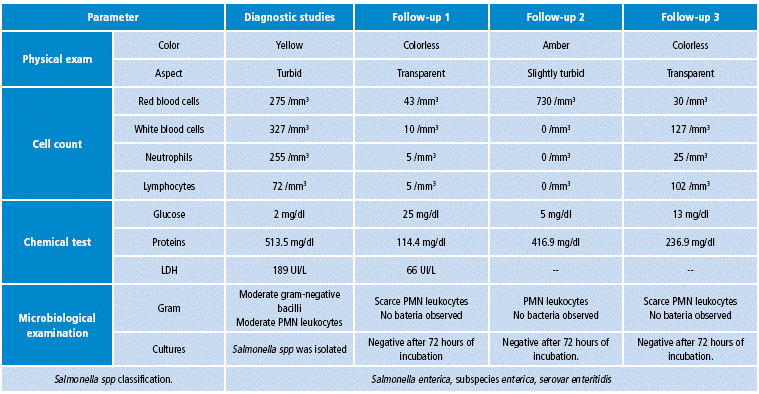
Source: Own elaboration based on the data obtained in the study.
In the critical care unit, evolution was torpid since a septic shock requiring inotropic support with milrinone at a maintenance dose of 0.4 mcg/kg/min was documented. After a CSF study report, a stool culture was performed, in which enteric pathogens compatible with Salmonella or Shigella were not isolated. However, two days later, isolation of Salmonella spp was reported in cultures of cerebrospinal fluid and blood, of a strain sensitive to ampicillin, ceftriaxone, imipenem, meropenem and trimethoprim-sulfamethoxazole, so meropenem was suspended after two days of treatment and antibiotic treatment was readjusted to ceftriaxone dose of 100 mg/kg/day.
In the critical care unit, evolution was torpid since a septic shock requiring inotropic support with milrinone at a maintenance dose of 0.4 mcg/kg/min was documented. After a CSF study report, a stool culture was performed, in which enteric pathogens compatible with Salmonella or Shigella were not isolated. However, two days later, isolation of Salmonella spp was reported in cultures of cerebrospinal fluid and blood, of a strain sensitive to ampicillin, ceftriaxone, imipenem, meropenem and trimethoprim -sulfamethoxazole, so meropenem was suspended after two days of treatment and antibiotic treatment was readjusted to ceftriaxone dose of 100 mg/kg/day.
Isolated Salmonella spp. was sent to an external laboratory for classification and was reported as Salmonella enteritidis. Meanwhile, a first simple and contrasting computerized axial tomography (CAT) was performed, concluding that it was normal. The patient remained hypotensive, with inotropic, tachycardia and tachypnea requirement, with metabolic acidosis evidenced in arterial blood gases and fever peaks; a five day course of antibiotic treatment with ceftriaxone was concluded with stationary evolution, period in which the patient also had seizures characterized by isolated episodes of gaze deviation, tachycardia and associated peripheral oxygen desaturation, initially managed with phenobarbital at a dose of 7 mg/kg/day without adequate clinical response.
Based on this situation, concomitant treatment with phenytoin at a dose of 5 mg/kg/day was indicated; meropenem was restarted and control cultures in blood, CSF and urine were taken. CSF control studies reported hypoglycorrhachia and hyperproteinorrachia within the ranges of bacterial meningitis, with fluctuating values of polymorphonuclear and leukocytes (Table 2). After 10 days of antibiotic treatment, a control simple and contrastig brain CT was performed, which reported meningeal vascular reinforcement and right frontotemporal and left frontal subdural effusions, consistent with clinical findings of acute meningitis (Figure 1). Nevertheless, due to clinical deterioration, the possibility of nosocomial gram-positive bacterial coinfection by germs was considered, so vancomycin was added to the antibiotic treatment at a dose of 60 mg/kg/day. Vancomycin was suspended five days later, after 72 hours without fever, within th context of negative control blood cultures, urine culture and CSF culture.
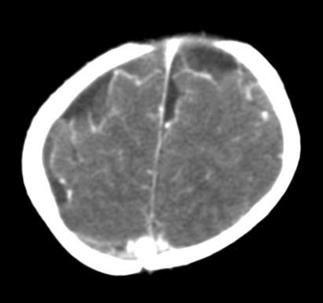
Figure 1 Simple and contrasting control CAT scan on the 10th day of antibiotic treatment. Source: Own elaboration based on the data obtained in the study.
Another simple and contrasting control brain CT scan performed at day 12 of treatment reported bilateral frontoparietal subdural collections, which suggested subdural empyema (Figure 2). A transfontanelar ultrasound was performed and then reported as normal. For this reason, performing a transfontanelar puncture was suggested by neurosurgery, but it was not performed due to favorable evolution.
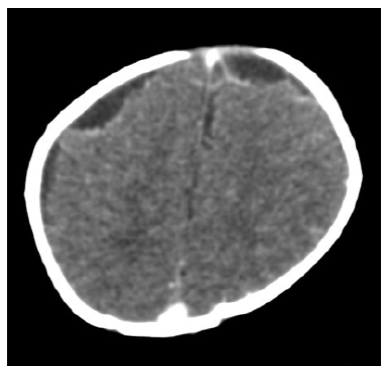
Figure 2 Simple and contrasting control CAT scan on the 12th day of antibiotic treatment. Source: Own elaboration based on the data obtained in the study.
After 19 days of combined antibiotic treatment that included ceftriaxone, meropenem and vancomycin, the infant gained clinical improvement obtained by limiting the convulsive episodes, as well as reaching hemodynamic and ventilatory compensation, and was transferred from the critical care unit to pediatrics hospitalization rooms with the aim of completing 21 days of antibiotic treatment with meropenem, which ended satisfactorily, without recurrence until discharge or after it. Three months after the acute event was solved, the patient returned to neurodevelopmental physical therapy and psychomotor prognosis was reserved, therefore, if neurodevelopmental goals are reached or if, instead, definitive neurologic sequelae are in place, can only be determined in the long-term.
Discussion
There are no case reports about Salmonella meningitis in the department of Cauca; epidemiological data are inconclusive and may be underreported. This clinical case is about a patient from the countryside, where pigs and chickens are raised in her home. It is known that Salmonella enteritidis colonizes the gastrointestinal tract of poultry and can be found on the surface of their eggs, so it is presumed that the transmission occurred through contact of the child with their caregivers. It should also be noted that the patient had no prior gastrointestinal conditions and the microorganism was not isolated in any stool sample. The literature has emphatically mentioned that the enteric picture precedes disseminated forms, so there are no reports about primary meningitis caused by Salmonella spp.
Given the difficulty in the design, there are no controlled clinical trials to consider antibiotic treatment of meningitis by Salmonella spp. and a consensus about treatment has not been reached 33, in consequence, its management is based on information documented by case reports or expert recommendations 8,34.
In the past, ampicillin, chloramphenicol and cotrimoxazole were used alone or combined for treatment, with not always favorable outcomes and a reported mortality rate associated to the treatment with ampicillin and/or chloramphenicol of up to 30% 6,35,39. With the arrival of third-generation cephalosporins, mortality rates and relapse of Salmonella meningitis have considerably decreased taking into account that Salmonella spp. meningitis resistance to these drugs is uncommon 35, but are occasionally reported 16,40.
Although ceftriaxone is an antibiotic with poor intracellular fluid penetration, its concentration in the cell is directly related to the concentration on the outside, so the effectiveness of the treatment depends on the time and the dose employed. Therefore, an intravenous treatment at high doses for at least four weeks must be assured 35. Currently, the American Academy of Pediatrics recommends the use of ceftriaxone or cefotaxime for four weeks or more, as lower courses of treatment have been associated with higher recurrence rates 16,41,42.
The resistance of Salmonella spp. to third-generation cephalosporins is rare, but the general resistance of Salmonella to antimicrobial begins to be a global problem in public health 43.
A descriptive study, which evaluated the susceptibility of 739 strains of Salmonella showed that serogroups A and D were sensitive to all drugs used (ampicillin, cotrimoxazole, chloramphenicol, ofloxacin, pefloxacin, norfloxacin, ciprofloxacin, ceftriaxone and cefotaxime). Serogroup B was sensitive to quinolones but resistant to ampicillin, chloramphenicol and trimethoprim-sulfamethoxazole in 78%, 83% and 54% of cases, respectively. Serogroup C had a resistance sensitivity profile similar to that of serogroup B. Finally, serogroup E was 100% sensitive to quinolones, but less sensitive to ampicillin, cotrimoxazole, ceftriaxone and cefotaxime at a 67-82% range 44. In this case report, although Salmonella strain was sensitive to third-generation cephalosporins in the antibiogram, clinical response using ceftriaxone was erratic, therefore, antibiotic treatment with meropenem was preferred.
On the other hand, the combination of third-generation cephalosporins with gentamicin, used in the initial treatment of gram-negative meningitis, may not be appropriate when it comes to intracellular facultative as Salmonella because gentamicin has poor penetration into intracellular fluids and does not penetrate the blood brain barrier well enough 16,45.The evidence of the use of carbapenems is insufficient, although satisfactory results have been reported in individual cases 16,38,39,41.
In the absence of pharmacological alternatives, the combination of ceftriaxone and ciprofloxacin, or ceftriaxone plus cefotaxime (if ciprofloxacin is contraindicated as in cases of newborn jaundice) can be considered for a minimum period of three weeks 16, even though that association has not been correlated to clear indications. This combination is not synergistic, but there is no evidence that pharmacological antagonism occurs between molecules 16,46. While the use of ciprofloxacin in children has been limited due to the possibility of arthropathy, the overall experience indicates that this is a rare event 47-49. There are reports that indicate the successful use of ciprofloxacin as monotherapy for Salmonella meningitis, but its combined use is suggested given the likelihood of resistance 16,40,50.
Conclusions
There is no consensus about antibiotic treatment of Salmonella meningitis. In the absence of clinical trials, cohort studies, and cases and controls, all recommendations come from case reports or advice from experts, models that have very low levels of evidence. Despite this, it is well recognized that ceftriaxone and cefotaxime are suitable alternatives, since the doses used have good penetration into the central nervous system and Salmonella resistance to these drugs is uncommon; therefore, they should be considered as the first choice of management.
The evidence on carbapenems is even more insufficient to recommend routine use; nonetheless, the case presented here suggests that meropenem can be an alternative to third-generation cephalosporins for the treatment of Salmonella spp meningitis.
Albeit with a low level of evidence, the available literature suggests that ampicillin should not be used empirically given the high rate of treatment failure. Similarly, fluoroquinolones, with no clear indication in the pediatric population, can be considered as pharmacological alternatives, especially in patients who do not have favorable clinical response or in relapse cases, but more studies with appropriate epidemiological design to support such recommendation are needed.
Finally, all isolation of Salmonella spp. in the cerebrospinal fluid requires antibiotic sensitivity studies that allow an adequate treatment.














