Introduction
Anopheles albitarsis F is a lineage characterized by sequences in the barcode region of mitochondrial DNA 1, ribosomal DNA ITS2 and fragments of the white gene 2. This species, together with Anopheles albitarsis I, are the only members of the Anopheles albitarsis Complex, found in the north of the natural barrier of the Amazon River. 2,3
In Colombia and Venezuela, An. albitarsis F has been registered as Anopheles marajoara4-6 because it has morphological characters similar to those of other members of the albitarsis Complex 7-9 and, because its molecular recognition is based on DNA fragments this has not allowed a clear separation between these species. 1,2,10 In the Colombian Orinoquia, An. albitarsis F is considered as an opportunistic species, with a wide distribution in urban and rural areas 11, behavioral resistance to insecticides 12, experimental infection by Plasmodium vivax13 and entomological inoculation rates of up to 5.1 of natural infection by Plasmodium falciparum.14,15 In this context, An. albitarsis F, in sympatry with Anopheles darlingi, may contribute to maintaining endemicity of malaria in the Orinoquia throughout the year. 16
The argyritarsis section of the subgenus Nyssorhynchus of Anopheles, that include all members of the albitarsis complex, is recognized by adults with hindtarsomere 5 entirely white, presence of two rows of white scales on first abdominal sternum, dark caudolateral scale tufts of abdomen, dark basal band of hindtarsomere 2; absence of sector pale spot (SP) in the Costa vein, and radial vein 3 (R3) with 1 to 3 dark spots. 17 The proportion of the basal dark band in hindtarsomere 2 (DS-III2) with respect to the total length of tarsomere (TaIII2) allows distinguishing two groups: one represented by Anopheles albitarsis s.s. and Anopheles deaneorum -characterized by having a range of 0.51-0.90-, and another conformed by Anopheles oryzalimnetes, An. marajoara and Anopheles janconnae, with a range of 0.28-0.63. 5 It appears that the ratio of DS-III2/Ta-III2 indirectly incorporates differences in body size from individuals in natural populations. 8 The proportion between prehumeral dark spot (PHD) and humeral pale spot (HP) in the Costa vein, with a range of 0.22-1.00, is a diagnostic character, considering that the fusion or not of the distal dark sectorial spots (DSD), subcostal pale spot (SCP) and dark preapical spot (PD) allows to recognize two different wing patterns. 6,18
In Colombia, although An. albitarsis I has been registered in the Atlantic Coast 2,19 and An. albitarsis F in the Orinoquia 2, studies of geometric morphometry and ITS2 (rDNA) question the separation of these two lineages 10. Since these species have not been described morphologically, entomological surveillance is complex, as it is based on the taxonomic determination of eggs, larvae, pupae or genitals of males from entomological series and isofamilies obtained from wild females that, due to their geographical distribution, can be related to each lineage or with mtDNA barcode sequences 1
In this context, morphometry of diagnostic characters in mosquitoes allows to infer biological information for natural populations, and geometric morphometric analysis, based on wing shape and size (individual wing conformation and population variation) 20,21, contributes to the taxonomic identification of the species and, therefore, to the accurate association of biological and ecological aspects. In anophelines, body size is relevant because it defines characteristics that determine its status as a malaria vector. It has been reported, for example, that males usually select females of larger body size for copulation 22,23 and that there is a positive correlation between wing size with respect to fecundity 24, daily survival rate 25, response to Plasmodium infection 26 and sensitivity to insecticides. 27
The objective of this study was to determine morphometric variations in the diagnostic characters of the wing and hind leg of An. albitarsis F females from two natural populations of the Colombian Orinoquía. The research was carried out using the characterization of the standardized nomenclature for the Costa wing scale spots; determination of morphometric variations in wings and, the proportion of basal dark spot of the hindtarsomere 2 with respect to total length hindtarsomere 2, by means of linear morphometry. Additionally, differences in body size by analysis of shape and wing size using geometric morphometric analysis were estimated.
Materials and methods
Entomological material
Specimens of An. albitarsis F from Puerto Carreño - Vichada and San José del Guaviare - Guaviare were included (Table 1).
Table 1 Geographical location of larvae and adults of Anopheles albitarsis F collected in two towns of the Colombian Orinoquia.
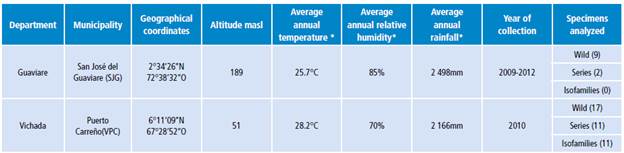
* Environmental information consulted in the Diccionario Geográfico de Colombia (Geographic Dictionary of Colombia) 28.
Source: Own elaboration based on the data obtained in the study.
Human landing catches were conducted indoors and outdoors of dwellings with malaria cases reported, for three consecutive nights each month, for nine months, between 18:00 and 06:00 hours, 50 minutes each hour. 29 Larvae and pupae were collected from larval habitats located close to the sites were adult females were caught. 30 Live wild-females were placed in plastic bottles labeled with collection information, provided with a 10% sugar solution, and transported in thermal boxes to laboratory conditions, where they were kept alive at a temperature of 26°±1°C, relative humidity of 75-80% and a photoperiod of 12L:12D. Isofamilies were obtained by forced mating as result of one leg and wing removed from each wild-female to obtain F1 offspring. Exuviae or larva IV and pupae were preserved in 70% ethanol labeled with the same code assigned to their wild adult parent. 31 Entomological series corresponded to the adults obtained from each immature form collected. Immature forms were placed in a five-ounce plastic cup with water from the same larval habitat; III and IV larvae were individualized to collect the emerged adult. 31 Larvae feeding was based on powdered light food for dogs (Purina® Dog Chow® Light), adding the same amount in milligrams for each series and isofamily. Wild females and males and females retrieved from immature forms maintained under controlled conditions were kept dry in Eppendorf tubes with a hole in the lid, arranged in plastic bags with silica gel.
All analyzes were carried out at the area of Insect Genetics of Economic Interest, at the Laboratory of Entomology, Faculty of Agricultural Sciences, Universidad Nacional de Colombia, Bogotá.
Protocols for mosquitoes collection procedures were approved by the Ethics Committee of the Faculty of Medicine of the Universidad Nacional de Colombia and by the protocol number 02-028 of the Institutional Review Board of the Wadsworth Center, NY State Department of Health, Albany, NY, USA. Mosquitoes sampling was supported by U.S. National Institutes of Health Grant (AI) R01 54139 "Malaria Vectors Biology in Brazil: Ecology & Genetics" and Universidad Nacional de Colombia, Bogotá, Faculty of Agricultural Sciences, Quipu code 201010012197.
Taxonomic identification was based on morphological characters 7,8, and, mtDNA-COI Barcode Region sequences. 1 gDNA was extracted from abdomens and the COI gene region was amplified using the LCO and HCO universal primers. PCR products were subjected to bidirectional sequencing and similarities with publicly available sequences on GenBank and Bold System (The Barcode of Life database) were assessed using BLAST (Basic Local Alignment Search Tool (http://blast.ncbi.nlm.nih.gov/Blast.cgi). 1
Measurements were made on 50 specimens (Table 1) of An. albitarsis F. The wings and legs taken from the right side of the body of each female were carefully removed and placed dry on microscope slides under a coverslip sealed with a transparent nail polish.
Linear morphometry analysis
The dorsal side of each wing was measured without scales, in millimeters, and based on the standard nomenclature for the Costal wing spots of the genus Anopheles as follows: basal pale + prehumeral pale (BP+PHP), prehumeral dark (PHD), humeral pale (HP), humeral dark (HD), presector pale (PSP), presector dark (PSD), sector pale (SP), proximal sector dark (PRSD), accessory sector pale (ASP), distal sector dark (DSD), subcostal pale (SCP), preapical dark (PD), preapical pale (PP), apical dark (AD) (6,18,32) (Figure 1A). The length (L) of each wing (at 30X) was measured from the alulae to the R3 intersection including the wing margin (Figure 1A). Ta-III2 and DS-III2 (Figure 1B) were measured for each hind leg and the DS-III2/TaIII2 proportion was estimated. All measurements were made using a Nikon® 5MZ 800 stereomicroscope with a ocular micrometer calibrated with a glass ruler to 0.01mm
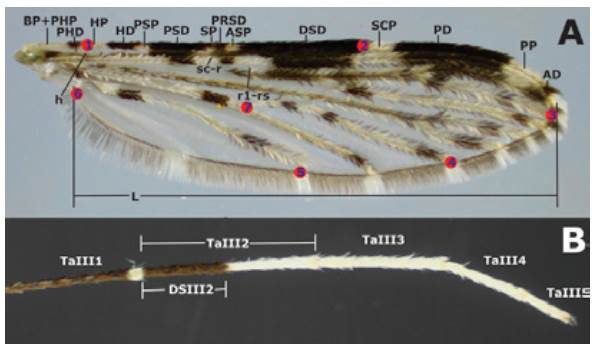
Source: Own elaboration based on the data obtained in the study.
Figure 1 Anopheles (Nyssorhynchus) albitarsis F. A) Wing with pattern I (no mergers) of Costa vein spots *; B) Tarsomeres of hind legt. * The red marks indicate the location of landmarks (LM) analyzed: LM1 intersection of the Humeral vein with Costa vein; LM2 intersection Subcosta vein with Costa vein; LM3 Radial 3 vein intersection with wing margin; LM4 intersection M3+4 vein with wing margin; LM5 Anal vein and wing margin intersection; LM6 alulae; LM7 anterior Cubital vein intersection with transverse mcu. t DS-III2/TaIII proportion is specified.
Costa vein spots measurements with respect to the wing length of each female (33) were standardized using equation [1] 34:
Where P is the transformed proportion of the length of the Costa vein spots with respect to the total length of the wing; X is the length of each Costa vein spot and L is the total wing length.
Statistical analysis was made based on the transformed values, taking the PHD/HP proportion of the wing spots, the total length of each wing and the DSIII2/TaIII2 proportion of the hind leg. Several Costa vein spot patterns have been recognized in Anopheles6, then, to unify the number of variables for statistical analysis, PSD, SP and PRSD spots (pattern I) measurements per female were added because they are fused in the pattern VI.
Normality tests were used 35, a multivariate analysis of variance and a test of Hotelling were performed for measuring the normalized spots. An analysis of variance for wing length was also carried out to establish possible significant differences between populations regarding the size of the specimens. According to source of specimens, three groups were established, one consisting of wild-females and another two, formed by females from entomological series and isofamilies. To describe the relationships between two or more compared groups in an algebraic way, a canonical discriminant analysis was carried out. 36 The statistical analyzes were made using the statistical programs InfoStat 37 and RWizard v.2.3. 38
Geometric morphometry analysis
Each wing evaluated by linear morphometry was photographed at 30X using a Nikon® DS-2Mv digital camera coupled to a Nikon® 5MZ 800 stereomicroscope. Seven anatomical landmarks at wing vein intersections, representing a polygon, covering the total area of the wing, were digitized in duplicate with TpsDig version 2.16 39 (Figure 1A). Since the wing did not have scales, all reference points corresponded to type I landmark. 40 Repeatability of landmark digitization was estimated by calculating the Rn index and both sets of digitized reference points were averaged to minimize the error associated with each specimen. 41
Wing shape was defined from two Procrustean coordinates for each landmark, while wing size corresponded to the size of the centroid expressed as the square root of the sum of the squared distances of each landmark to the centroid of the polygon. Landmarks of each specimen were scaled, translated and rotated by superposition with respect to the consensus configuration, using a Procrustes analysis. 42 This analysis allows to center each landmark configuration at the origin that is aligning all the landmarks configurations to their centroid in a way to eliminate the effect of position. Each landmark configuration is scaled to unit centroid size to eliminate the effect of size; and then they are rotated around the origin to minimize the summed square distance between homologous landmarks, in order to remove the effect of orientation.
Statistical analyzes were carried out using the Morpho J program. 43 For this, the file generated in TpsDig2 version 2.16 39 was imported with landmark coordinates and a graph was obtained using the RWizard software. 38 The normality of the data was verified 35, differences of size and wing shape were evaluated by means of an analysis of variance 44,45 and a discriminant analysis was performed with 10 000 permutations using Mahalanobis distances between groups as criteria for discrimination to determine possible differences in wing shape in each group studied. 46 The allometric effects, understood as the effects of size on the wing shape, were evaluated with a multivariate regression analysis of shape variables onto size, using the coordinates of each landmark with respect to the centroid. The statistical significance was estimated by non-parametric testing using 10 000 permutations. 47
Results
Linear morphometry analysis
The pattern VI of the Costa vein spots was the most predominant (74%, n=37), followed by the pattern I without merger of Costa vein spots (26%, n=13) (Figure 2).
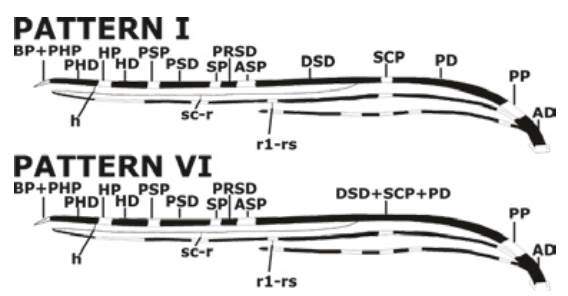
Source: Own elaboration based on the data obtained in the study.
Figure 2 Ideogram of the two patterns of the Costa vein spots found in natural populations of Anopheles albitarsis F in two towns of the Colombian Orinoquía.
The typical PHD/HP proportion for An. albitarsis s.l was represented by a 0.4-0.6 range in 29 specimens (58%) 5,8 However, a range of 1.17-1.19 was obtained for 49 specimens (98%) after standardizing the data by the equation [1], considering the length of each wing length and analyzing the mean (1.18) ± three times the standard deviation of the data distribution (3a=0.0105),DSIII2/TaIII2 proportion registered a range of 0.5-0.9 for 24% (n=12) of females 5,8, but when the mean (2σ=0.54) ± two times the standard deviation (0.08) of its distribution was estimated, 98% (n=49) of the specimens were represented by the 0.38-0.54 range.
Largest females (2.96 mm) corresponded to wild-females, followed by those obtained from entomological series (2.74 mm) and isofamilies (2.50 mm). Those three groups were significantly different (F=29.67, gl=2, p<0.001). Wild-females from Puerto Carreño (2.96 mm) had a greater wing size (F=5.00, gl=1, p=0.04) than wild-females of San José del Guaviare (2.79mm).
Not significant differences between populations (F=1.23, gl=14, p=0.43) were found when the lengths of the Costa vein spots and the proportions between them, considered as diagnostic, were evaluated. However, when each group was analyzed separately (wild females, series and isofamilies), An. albitarsis F population from Puerto Carreño showed significant differences (F=3.60, gl=26, p=0.001) in the Costa vein spots length and in the diagnostic proportions used for taxonomic identification.
Discriminant analysis of principal components for the wing length variables and the PSD, SP, PRSD and PD spots, allowed correct identification of 100% of females for each group analyzed (Figure 3).
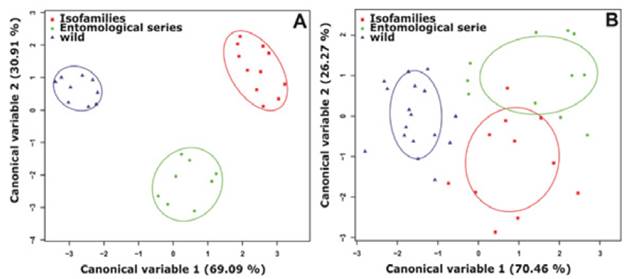
Source: Own elaboration based on the data obtained in the study.
Figure 3 Discriminant analysis for Anopheles albitarsis F females from Puerto Carreño, Vichada. Each dot represents each female analyzed while the ellipses indicate groups according to the source of specimens. A) Linear morphometry. B) Geometric morphometry. Software RWizard 2.3. 38
Geometric morphometry
Repeatability values for landmarks digitization were found for both, the Procrustes coordinates (Rn=0.98) and the centroid size (Rn=0.99). The analysis of variance did not show significant differences either in size (F=0.03, gl=1, p=0.87) nor in wing shape (F=1.04, gL=10, p=0.18) for wild-females according to their geographical origin. However, as in the case of wing length, wing size of An. albitarsis F from Puerto Carreño (2.64mm) was larger than wing size of the population from San José del Guaviare (2.63mm).
According to the source of each group analyzed (wild-females, series, and isofamilies), Puerto Carreño groups (n=39) showed significant differences, both in size (F=3.65, gl=2, p=0.036), and wing shape (F=7.02, gl=20, p<0.0001) where wild-females were larger (2.64mm) than those obtained from entomological series (2.52mm) and isofamilies (2.43mm). Discriminant analysis allows identifying correctly 86% (n=43) of the females from Puerto Carreño based on landmarks 2, 4, 6 and 7, particularly (Figure 4). Wing shape registered differences in the axillary, anal, cubital and radial wing regions of wild-females and those obtained by series and isofamilies. In addition, a comparison between consensus configurations was observed for all three groups (Figure 4).
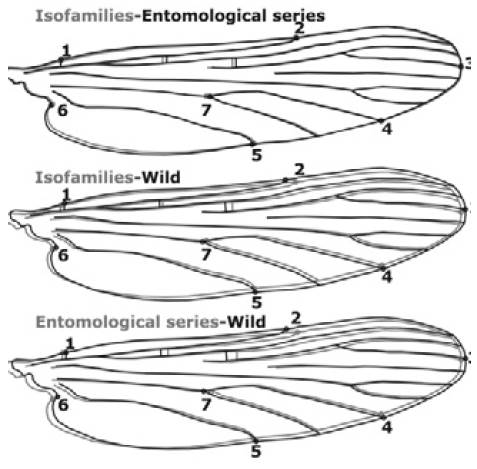
Source: Own elaboration based on the data obtained in the study.
Figure 4 Graphic representation of paired groups showing the variation of wing shape in Anopheless albitarsis F of Puerto Carreño, Vichada. Software MorphoJ 43.
Regression analysis of the variables associated with shape and wing size showed evidence of allometric effects (p <0.0001) with a prediction of 33.7%. Since almost identical results were found when regression residuals were used in a new discriminant analysis to correct allometric effects, the first analysis was considered.
Discussion
An. albitarsis F from the Colombian Orinoquia showed slight phenotypic variation in wings, represented by two of the Costa vein spot patterns like 12 Venezuelan populations of An. marajoara6 as An. albitarsis F or An. albitarsis I 6, two lineages only reported in the north of the Amazon River.
Differences in the length of the Costa vein spots, wing shape and centroid size could be influenced by breeding conditions. Under controlled conditions, a nutritional imbalance derived from artificial diets 27,48, population density, natural lighting and changes in water temperature are aspects that define the fitness of entomological series and isofamilies 49. In natural conditions, wild females develop under different conditions. The Colombian Orinoquia has a monomodal rainfall regime 50 in which important rivers such as the Orinoco, the Meta, and the Guaviare rivers generate runoff that provides nutrients to all mosquito larval habitats. These habitats are permanently exposed to the sun 51, so natural light determines the conversion of essential fatty acids and contributes to the quality and quantity of essential micronutrients for the development of immature forms of anophelines. 52,53 In consequence, in natural populations, the genetic background together with abiotic factors, defined by latitude and ecological characteristics, may play a fundamental role in the phenotypic expression of these traits for the species.
Wing length in mosquitoes of the genus Anopheles constitutes an indirect estimator of body size 20, a characteristic associated with their status as malaria vector. This study revealed that wild females from Puerto Carreño, Vichada have larger relative body size than the wild females from San José del Guaviare, Guaviare. This is interesting since Puerto Carreño populations have been found to be naturally infected by P. falciparum, reaching entomological inoculation rates of up to 5.1 54, while no natural infection by human malaria parasites has been found in populations of An. albitarsis F from San José del Guaviare. 55
As in other mosquito species, the ratio of wing size may be involved in fitness through the preference for copulation 21,22, increased fecundity 24 and, the ability to search for a good quality blood source. 25 In general, for anophelines, larger females have a higher rate of bite in humans and a higher rate of survival, which favors infection with Plasmodium spp.52,56. In this context, in the Orinoquia Region of Colombia, An. albitarsis F may act as an auxiliary or regional vector, contributing to maintaining malaria endemicity, even when populations of the primary vector An. darlingi are very low. 11
It is clear that body size influences the vectorial capacity of mosquitoes ofthe genus Anopheles; for this reason, it is very important to analyze the wing length as an indirect estimator of body size for taxonomic identification of the lineage of An. albitarsis F, and associated bionomic aspects ofnatural populations. Based on linear and geometric morphometric analyzes, this study proposes the main diagnostic characters for An. albitarsis F, a range of 1.17-1.19 for the PHD/HP proportion of the Costa vein spots and, the range of 0.38-0.54 for the proportion of DSIII2/TaIII2 of hindtarsomere 2 (Table 2). Analyzing these range values, in the Argyritarsis Section, An. albitarsis F would be closer to An. oryzalimnetes and An. janconnae than An. marajoara, as this lineage has been denominated in previous studies with specimens from Venezuela and Colombia. 5 However, more studies for these diagnostic characters with a greater sample size and that include samples of both lineages, An. albitarsis F and An. albitarsis I, are required.
Table 2 Diagnostic character ranges for natural populations of Anopheles albitarsis F for Colombia, Brazil and Venezuela.
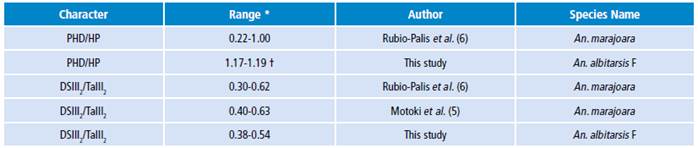
* Range obtained by using equation [1].
† An. albitarsis F has been denominated as An. marajoara in other studies.
Source: Own elaboration based on the data obtained in the study.
For members of An. albitarsis Complex, it is important to normalize [equation 1] the measurements of each Costal vein spots with respect to the wing length (body size) of each specimen analyzed for morphometry studies. This allows a better association between taxonomic identification status and biological and ecological characteristics based on their geographical location and epidemiological scenarios. Ranges for DSIII2/TaIII2 proportion found in this study overlap with ranges for this proportion reported in natural populations of An. marajoara4-6 without molecular confirmation 1,2,10, so it is likely that, in fact, this means a synonymy and the ranges observed for An. marajoara are actually ranges for An. albitarsis F, which would support the range proposed in this study (Table 2).
The study of diagnostic characters based on linear and geometric morphometry helps to define the identity of lineages that do not yet have a morphological description, as is the case of An. albitarsis F, and offers indirect information on some aspects of its biology, which are fundamental for understanding their role as malaria vectors. The sample size for this study was limited by molecular confirmation, so evaluating the same parameters with a greater number of individuals, whose sample size shall be representative for wild populations of An. albitarsis F and other members of the An. albitarsis Complex, is suggested. Since latitude and abiotic factors determine wing size and shape in mosquitoes 57, populations from other locations should be included throughout their geographical distributions and in different collection years that include El Niño-Southern Oscillation (ENSO) for linear and geometric morphological analysis of diagnostic characters used for taxonomic identification.
Conclusion
In natural populations, females of An. albitarsis F can vary in size according to their geographical and ecological origin. Biotic and abiotic factors associated with obtaining entomological series and isofamilies under controlled conditions, as a strategy for taxonomic determination based on the morphological characters of their associated stages, define the differences in body size and wing shape with respect to wild -females. This study presents new ranges for diagnostic characters used for the taxonomic identification of An. albitarsis F in Colombia.















