Introduction
Total hip arthroplasty (THA) is an effective method to treat severe degenerative and post-traumatic diseases at the hip joint. However, THA sometimes fails due to prosthesis design, surgical technique, fixation method, early infections, or late aseptic loosening (AL). Besides, patients with such conditions need revision surgery, which leads to risk of complications and functionality problems. 1,2
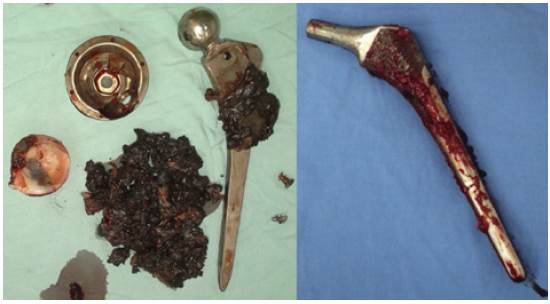
A tissue component with high concentration of monocytes-macrophages, fibroblasts, vascular endothelial cells, and polymorphonuclear neutrophils can be observed in loosened boneacetabular cup interfaces and pseudocapsular tissue. 3 Such cells induce osteolysis that appears as diffuse cortical emaciation (osteoclasts) or focal lesions (osteoblasts). Pain is frequent and caused mainly by the loosening process and not by infection. 4 Debris detachment (Figure 1) and subsequent biologic response lead to loosening.
Source: Document obtained during the conduct of the study.
Figure 1 Hip replacement arthroplasties. Left: AL with dark periprosthetic tissue. Right: biomechanical loosening.
This paper aims to describe the mechanisms of biological interaction of the molecules that promote total hip prosthesis loosening, specifically, interleukins, cathepsins, prostaglandin E2 and matrix metalloproteinases. Furthermore, a scheme of the biochemical loosening of the hip prosthesis is presented.
Materials and methods
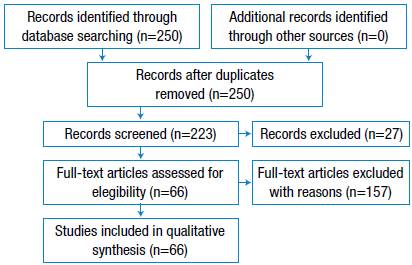
A comprehensive online search was performed in the PUBMED and SCOPUS databases. Only original articles and clinical cases were analyzed, whereas conference articles and surveys were excluded. The nature of the search implied an open filter for the initial publication date; the first article found dates back to 1979, while the latest publication date is June 2017. The search period ranged between 2015 and 2017. The language of the articles was limited to English and Spanish. The following MeSH terms were used for the search: [hip prosthesis loosening] OR [aseptic loosening] OR [hip arthroplasty failure] AND [cytokines]. Only articles on the following subject areas were included: Medicine, Engineering, Biochemistry, Genetics, Molecular Biology, Materials Science, Immunology, Pharmacology, Toxicology and Pharmaceutics. Engineering-related documents included only reviews and book chapters. Eligibility criteria of life-sciences-related articles included studies carried out in human groups and murine models. Furthermore, only documents focused on the description of biological loosening and its relationship with mechanical events were included for qualitative synthesis (Figure 2).
Results
Effect of the material on THA performance
Charnley 6 showed that living bone could grow in contact with polymers such as polymethyl-methacrylate (PMMA). Block-PMMA is in contact with the bone for long periods without adverse reactions 7, but its particles induce a macrophage response followed by the formation of giant cells. This process is highly dependent on the size and amount of PMMA particles. Enzymes cannot degrade ingested PMMA, therefore, the material accumulates in the cells. The saturated cells die, thus producing chronic inflammation, necrosis, osteolysis, and implant loosening (Figure 3). Such process has been called "cement disease", "prosthetic synovitis", "detritus disease", and even "no-cement disease". 8,9
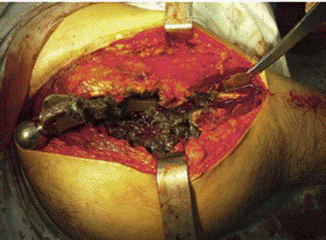
Source: Document obtained during the conduct of the study.
Figure 3 THA revision caused by AL (see the dark necrotic tissue).
Since the 1960's, and until 1975, the most commonly used pair in THA was metal-metal, even though it produces high rates of loosening and sensitivity. The level of metallic particles in synovial fluid is higher in loose prosthesis than in well-fixed ones. 9 Since 1975, the metal-polyethylene pair has been used; however, polyethylene (PE) generates osteolysis with a high particle delivery rate. 10 In the 1980's, the metal-metal pair returned with a refurbished composition. Several authors reported an intrinsic connection between the progressive loosening of acetabular cups and PE wear. 11 This complication led to use ultra-high molecular weight PE (UHMWPE), highly cross-linked PE, and ceramic-ceramic pairs. 12
Although metallic debris can induce cellular death, most of inflammatory mediator releases are caused by the stimulation of viable cells. Cellular response to polymeric, ceramic, and metallic particles has been studied in patients with osteoarthritis, rheumatoid arthritis, and avascular necrosis, all of them with THA. 8 The literature suggests that the process involves an inflammatory reaction and severe immune response against the material.
Cathepsins
Cathepsins (proteolytic enzymes) are present in the synovial membrane. Cathepsin K (CTSK) is the predominant cysteine protease in bone turnover and it is mediated by osteoclasts. When the tissueimplant interface is acidified by giant cells, CTSK activates, thus leading to dissociation of the hydroxyapatite from the periprosthetic cortical bone. This process is followed by the degradation of type I collagen. 13-16
The production of CTSK and the receptor activator for nuclear factor kB ligand (RANKL) increase when fibroblasts are stimulated by tumor necrosis factor alpha (TNF-α), interleukins 1β, 6 and 11, and vitamin D3. These molecules are involved in the genesis of periprosthetic collagen and bone destruction. 15
The expression of CTSK in monocyte-macrophages is accompanied by in vitro resorption of carbonated calcium phosphates. In patients with loosened prosthesis, high collagen degradation is observed due to CTSK and metalloproteinase 13 activity. 14 Although the most studied cathepsin in AL is CTSK, other cathepsins, such as cathepsin G (CTSG), B, D, and L, are involved in the process.
CTSG is a modulator of inflammation and connective tissue remodeling. 3,17 This enzyme is located in the endocytic compartments of primary human B cells, myeloid dendritic cells, and murine microglial cells. 18 In loosened bone-acetabular cup interfaces and pseudocapsular tissue, CTSG is present in approximately 70% of monocytes-macrophages and in around 50% of fibroblasts. 3 However, the action mechanism is not completely understood.
In patients with AL, the prevalence of cathepsins B, D, and L at synovial periprosthetic interfaces, rheumatoid arthritis and primary osteoarthritis is 100%, 62.14%, and 77.1%, respectively. 19
Cytokines
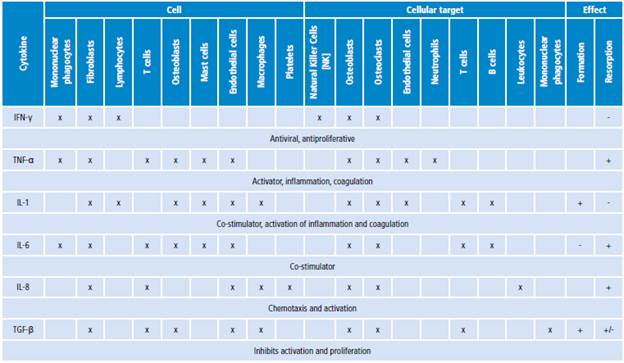
Cytokines are low molecular weight proteins that act as mediators in natural immune processes such as regulation of lymphocyte functions, activation of inflammatory cells, and stimulation of hematopoiesis. During the natural immune process, cytokines are delivered by monocytes and macrophages. Cytokines (Table 1) include interferon gamma (IFN-γ), tumor necrosis factor alpha (TNF-α), and interleukins 1, 6 and 8 (IL-1, IL-6, IL-8). 20
Interferon gamma (IFN-γ)
IFN-γ is the most important factor in the activation of macrophages; it acts as co-stimulator of other cytokines (IL-1, IL-6 and TNF-α) and surface molecules 21, and may activate the antigen-presenting cells, which become more efficient in presenting antigens. 22
IFN-γ inhibits bone resorption and does not show concentration changes in membranes of cemented or cementless prostheses, with or without osteolysis. 23 Patients with moderate osteolysis have increased IFN-γ concentration in presence of metallic alloys in the femoral neck or head of the prosthesis. 19 Nevertheless, immune reaction depends not only on the concentration of metallic particles but also on ceramic or polymeric particles. For example, the presence of UHMWPE activates the NKT cells, which modulate the pro-inflammatory response of IFN-γ and the subsequent activation of M1 macrophages. This leads to tissue damage and periprosthetic osteolysis. 24
Tumor necrosis factor-α (TNF-α)
TNFs are functionally related to IL-1, IL-6, and IL-8. 23 TNF-α has a key role during the inflammation process as it activates neutrophils, eosinophils and mononuclear phagocytes to eliminate bacteria. Furthermore, TNF-α is a powerful inductor of other cytokines and stimulates other phagocytes for the production of IL-1, IL-6 and IL-8. 2,20
TNF-α is a potent promoter of bone and cartilage resorption that stimulates the proliferation of osteoclast progenitors, the decrease in collagen synthesis, and the activity of alkaline phosphatases in osteoblasts. 25 The lock of the TNF-α activity inhibits both osteoclast differentiation and osteolysis induced by wear debris 26,27.
TNF-α is found in fibroblasts and vascular endothelial cells of the periprosthetic tissue, but mainly in the macrophages of the surrounding areas with implant debris and in the synovial membrane of the interface tissue around the femoral stem. 28 The interaction mechanism at the molecular level is unclear. 29
The TNF family includes RANKL, an activator that can be expressed in surfaces of myeloid progenitor cells, pre-osteoclasts, mature osteoclasts, and T and B lymphocytes. The RANKL/RANK complex is an important promoter of osteoclasts. Tissues with periprosthetic osteolysis are rich in both TNF-α and RANKL. 30-32
Interleukins 1, 6, and 8 (IL-1, 6, and 8)
IL-1 was the first cytokine with a well-known function in bone resorption mediated by osteoclasts, and many studies have demonstrated its important role in HP loosening. An IL-1 blockade leads to partial inhibition of inflammation and differentiation of osteoclasts 33,34, while the presence of polymorphism in the IL-1 antagonist gen is frequently associated with AL. 35 The levels of RANKL, TNF-α and IL-1P in plasma have an incremental pattern in patients with early loosening, in contrast to late loosening. 36
IL-6 is produced by many kinds of cells, including macrophages, multipotent stem cells, osteoblasts, and mastocytes. 37 The effects of IL-6 on bones have been controversial, although most studies indicate that it stimulates the formation of osteoclasts and bone resorption through an increase in RANKL production. 38-40) IL-6 has a similar behavior to IL-1 and TNF-α. These three cytokines can induce the production of one another and result in a synergistic response when they are added together to assay systems. 22,41,42 The release of IL-6 and PGE2 is increased by osteoblasts after the addition of IL-1, TNF-α, metallic particles, metallic ions and macrophages in vitro. 43
In patients with femoral osteolysis, the presence of IL-6, IL-1 and TNF-α is notorious in the interfacial membranes around the HP. 44 A systemic increase in IL-6, IL-8 and IL-16 induces an accelerated inflammatory response with AL. 45 IL-6 and IL-8 could be the promoters of the final stage of osteolysis, whereas TNF-α and IL-1 do not have an important role at that stage. 46
IL-8 is a mediator of inflammatory processes 20,22 that attaches mononuclear and polymorphonuclear cells to the inflammation site. This process enhances the release rate of degradative enzymes (e.g., collagenase), PGE-2, IL-1, and TNF-α. All of these molecules are frequently detected in revision surgeries. 47 IL-8 is produced by monocytes, T cells, fibroblasts, endothelial cells, and keratinocytes as a result of stimulation by IL-1 and TNF-α. IL-8 is found in periprosthetic tissue accompanied by TNF-α, transforming growth factor-β, IL-1, IL-6 and IL-11. 48 The amount of IL-8 in synovial fluid is higher in patients with replacement arthroplasty than in patients with primary arthroplasty. 47,49 In fact, IL-8 concentration in serum can serve as a marker for periprosthetic osteolysis. 50
An important finding is that the concentration of IL-6 and IL-8 is higher in the synovial fluid of patients with THA replacement caused by AL compared to patients with HP fracture, who have a more significant concentration of the IL-1 isoform, IL-1β. 42
Different alloys can stimulate the production of interleukins 23 in different ways. For example, Ti-Al-V induces the release of IL-1 and IL-6, whereas the Co-Cr alloy does not. 24
Transforming growth factor beta (TGF-β)
TGF-β is involved in healing-inflammation cascades 22 and inhibits bone resorption through the inhibition of differentiation and proliferation of osteoclast precursors. TGF-β affects osteoblasts and stimulates bone growth 23, although its action mechanism is not completely clear. The effect of TGF-β is unrelated to the use of a specific alloy or cement.
Patients with AL have a lower concentration of TGF-β2 than those with permanent prosthesis. The latter present a higher concentration of this protein compared to patients with primary arthroplasty. 51
Possibly, the mere presence of the implant leads to local release of TGF-β by the osteoblasts. The growth of low-quality bone could be related to an insufficient increase in TGF-β, as there is an inverse relation between the concentration of growth factors and the loosening rate. 51
Prostaglandin E2 (PGE2)
The effect of PGE2 in HP loosening has been studied since the 1980's, when the presence of cells in the synovial bone-implant interface with high concentration of PGE2 and collagenases was proven. 52
PGE2 is an important intracellular messenger in the osteolytic cascade produced by the presence of implant debris. 22 Similarly to IL-1, PGE2 is an important promoter of bone resorption through the stimulation of osteoclasts. However, at low concentrations, PGE2 inhibits bone resorption and the stimulation of osteoblasts. The release of PGE2 by osteoblasts increases when IL-1, TNF-α, metallic particles and ions are present. 43
The release ofPGE2 mediated by osteoblasts is favored by wrinkled surfaces 53, although some studies on the effects of the material composition are contradictory. 43,54 However, the role of PGE2 in the loosening of the HP is sufficiently supported by complex mechanisms that involve cytokines and matrix metalloproteinases. 55,56
Matrix metalloproteinases (MMP)
A misbalance between MMPs and tissue inhibitors of MMPs (TIMPs) contributes to prosthetic loosening. 57 Nevertheless, the specific mechanism whereby these enzymes are controlled in the periprosthetic medium is unknown.
Interstitial MMPs like collagenases (MMP-1, MMP-13), gelatinases (MMP-2, MMP-9) and stromelysin (MMP-3) are involved in loosening processes through the degradation of components from the extracellular matrix. 55 Collagenases are primers of connective tissue remodeling that degrade type I and III collagen and facilitate bone resorption by removing the osteoid film from the calcified bone. 55
In periprosthetic media, MMP-1 is found in monocytes-macrophages, fibroblasts, and vascular endothelial cells in both the interface of the bone-acetabular component and pseudocapsular tissue. MMP-8 is occasionally located in polymorphonuclear monocytes. MMP-2, MMP-3, and MMP-9 are found in cells located at the interface and pseudocapsular tissue 58, which demonstrates the role of MMP-1, 2, 3 and 9 in HP loosening.
Ex vivo assays have shown that TIMP-1 is found in periprosthetic tissue and fluid samples of loosened prosthesis. At low TIMP-1 concentrations, a high percentage of degradation of the synthetic substrate is observed, which leads to collagenolytic and gelatinolytic activity on the interface tissue. This explains the weakness of the periprosthetic connective tissue that results in HP loosening. 59
Studies about the expression pattern of mRNA in different kinds of MMP show that the amount of MMPs is related to HP loosening.
This occurs because MMPs generate pathological degradation of the extracellular matrix and connective tissue around the prosthesis. 60 Furthermore, there is evidence that suggests that polymorphisms in the MMP-1 gen lead to HP loosening and can be associated with genetic predisposition. 61
Discussion
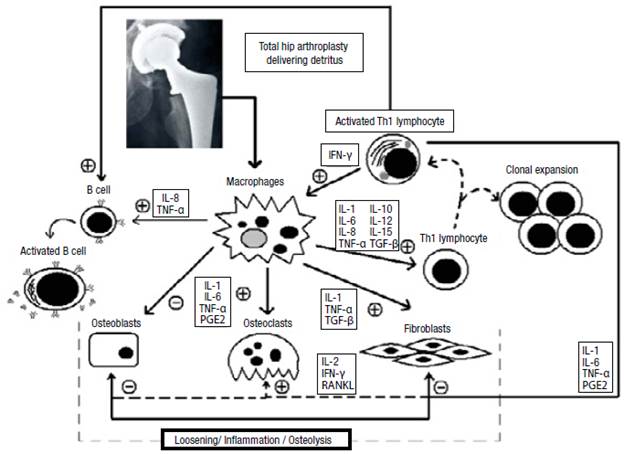
HP loosening may be caused by biological, technical, or biomechanical factors. 62 Although the exact mechanism whereby the complete process occurs is not well-known, the most widely accepted hypothesis is that the sole presence of the foreign material leads to accumulation of macrophages in the periprosthetic tissue, which absorbs the wear debris of the prosthesis and cement.
The detritus activates the macrophages to produce proinflammatory cytokines, which are potent stimulators of bone resorption, through direct and indirect stimulation. Such cytokines stimulate local factors that increase the bone resorption rate. They also induce the production of pro-collagenases by connective tissue cells, thus activating osteoclasts. Osteolysis associated with HP loosening is produced by osteoclasts and enzymes (cytokines, growth factors and prostaglandins).
An abnormal immune response may destroy the periprosthetic tissue. When the immune system, endothelial cells, fibroblasts, osteoblasts, and osteoclasts are activated, they synthesize the cytokines involved in inflammatory and osteolytic processes. MMPs may also lead to the destruction of the periprosthetic tissue.
Although it is not possible to establish a deterministic model for the release of the abovementioned proteins, there is a general mechanism in which the cytokines and other molecules interact due to prosthesis loosening. This mechanism presents biochemical cascades with positive and negative feedback (Figure 4).
There are potential treatments for AL that include biochemical agents such as bisphosphonates (e.g., calcium alendronate, risedronate and zoledronic acid) that inhibit bone resorption and interact with mature osteoclasts. Likewise, RANKL antagonists, such as Denosumab, prevent bone resorption. The difference with bisphosphonates is that RANKL antagonists have the unique ability to inhibit directly the formation, activation and survival of osteoclasts. 63
Additionally, non-steroid anti-inflammatory drugs (NSAIDs), like celecoxib, are used against rheumatoid arthritis and osteoarthritis. The anti-inflammatory antagonists of cytokines, such as TNF antagonists (Etanercept, Infliximab Adalimumab, and Anakinra), are used for psoriatic and rheumatoid arthritis, and have the potential to prevent the dramatic outcome of HP loosening.
The problem of using antagonists and anti-inflammatory drugs is their immune-suppressor effect. However, this does not hold true for RANKL antagonists. Because of this and their high specificity, RANKL antagonists are the best candidates for non-surgical treatment of patients with loosened HP. 63 Another possible strategy to avoid the loosening process could be applied on primary THA: introducing antibiotics and NSAIDs in the bone cement. 64
The molecules that have been studied as possible biomarkers are IL-1, IL-6 and IL-8. All of them have shown important activity in serum and synovial liquid, although more studies are needed to demonstrate their capacity. 42,65,66
Another possible treatment against prosthesis loosening is gene therapy. Although studies are limited and preliminary, certain polymorphisms that predispose to loosening have been detected. 61,67 Furthermore, genetic markers of inflammatory proteins involved in loosening are associated with those polymorphisms.
Conclusions
The described mechanisms establish a close relationship between hip prosthesis loosening and the release of enzymes and cytokines by immune system cells. Thus, the authors propose to name this process "enzymes and cytokines disease".
The trend in loosening treatments is to strive for early detection based on the patient's predisposition to prosthesis failure. In other words, there is a need for a preventive approach in the orthopedic technique that involves not only biomechanical and physiological analysis but also genetic variables.
In the future, AL prevention will be focused on the production of enzyme antagonists, cytokine receptor blockers, collagenase inhibitors, and the development of new biomarkers that enable early detection of the problem.