Introduction
Obstructive sleep apnea-hypopnea syndrome (OSAHS) is a frequent entity with an estimated prevalence of 3-7% in the adult population. 1 This condition is characterized by recurrent airway collapses during sleep and is associated with cardiovascular complications such as stroke 2, acute myocardial infarction and hypertension 3, as well as with increased risk of traffic accidents 4 and significant deterioration in quality of life. 5 The standard diagnostic study is polysomnography (PSG), which includes video recording, identification of different sleep stages and their distribution throughout the study, heart rate, chin and anterior tibial muscles activity, position during the study through position sensor and video recording, presence of snoring, and apneas and/or hypopneas and their association with oxygen saturation. Based on these results, the severity of OSAHS and the fragmentation of sleep that is derived from this respiratory alteration are determined.
In patients with moderate to severe OSAHS, first-line treatment involves continuous positive airway pressure (CPAP). 5 A second PSG is required to determine the optimal pressure to correct OSAHS. This treatment improves sleep quality and quality of life, and reduces daytime sleepiness. 6,7 Furthermore, CPAP may also have a positive impact on reducing cardiovascular risk. 8,9 The conventional approach that requires two PSG -one for diagnosis and another for CPAP titration- is costly, time consuming, and delays the initiation of CPAP treatment for this group of patients. 10
The combined use of PSG and CPAP titration in one night, known as split-night polysomnopraphy (SNPSG), is an alternative that allows for proper diagnosis and treatment in less time, thus optimizing the use of resources and patient comfort. 11,12 Elshaug et al.13 achieved a 15% reduction of the time to start treatment in patients with severe OSAHS, which accelerated timely care and management of this group of patients. This procedure has sufficient diagnostic precision for patients with moderate and severe OSAHS 14 and is used in up to 20% of patients attending sleep laboratories in the United States. 15 In Colombia, however, there is no research indicating the usefulness of SNPSG. The objective of this study is to describe the split- night polysomnography procedure performed in the sleep laboratory of the Hospital Universitario de la Fundación Santa Fe de Bogotá (HUFSFB) and to assess its performance in the diagnosis and CPAP titration.
Materials and methods
A retrospective, observational and longitudinal study was conducted to describe and analyze the characteristics of the SNPSG studies performed in the HUFSFB sleep laboratory between 2013-2014. The study was approved by the HUFSFB Corporate Research Ethics Committee through Act No. 2 of February 8, 2016. In addition, this study complied with the ethical principles for medical research involving human beings of the Declaration of Helsinki 16 and Resolution 8430 of1993 of the Colombian Ministry of Health and Social Protection. 17 All SNPSG results of patients over the age of 18 referred to the sleep laboratory for split-night study were included. Patients with incomplete records, those who had previous CPAP treatment and attended the hospital for pressure titration control and those who rejected the use of CPAP since the beginning of the study were excluded.
Sample size was calculated to find repeated samples in the same group using the prevalence of apnea in the general population before CPAP (5%) and the prevalence of apnea after an unsuccessful overnight study (12%) as parameters 12,18, with a beta error of 0.20 and alpha error of 0.05 for a total of 194 patients. All patients meeting the inclusion criteria were selected until sample size was exceeded. The subjects studied in the sleep laboratory were referred by specialists in multiple areas such as internal medicine, neumology, otorhinolaryngology, neurology and obesity clinic. This implies that they had a high diagnostic suspicion before the test was made.
During this period, SNPSG was performed in all patients with Respironics Alice 5 of Respironics Inc., a brand that has been used in the institution since 1994 and is commonly used in sleep laboratories worldwide. The result was read by the same sleep medicine specialist. The PSG recorded electroencephalogram (EEG), electrooculogram (EOG), surface electromyography of the chin and anterior tibial muscles, electrocardiogram (ECG), airflow with pressure transducer by nasal cannula (Pro-Tech), chest and abdomen effort using plethysmography bands, oxygen saturation by pulse oximetry (Massimo), body position sensor, video and CPAP (Resmed) with remote control for pressure titration.
Before starting the titration study, patients had the opportunity to wear the CPAP mask at 4 cm pressure for 20 minutes to become familiar with the procedure. The diagnostic phase took place during the first 3 hours and pressure titration with CPAP was carried out in the second phase. The initial pressure of 4 cm was gradually increased according to patient tolerance. Care to ensure proper registration and accompaniment of patients was provided by personnel trained in polysomnography. CPAP pressure titration was based on the American Academy of Sleep Medicine (AASM) guidelines 19,20, which establish a titration time of no less than 3 hours during which a complete decrease in respiratory events during rapid eye movement (REM) and non-REM sleep is achieved, including REM sleep with the patient in supine position, abolition of snoring and correction of desaturation.
The SNPSG record was analyzed by a specialist in sleep disorders according to the parameters established by the AASM as follows. 19,20
Obstructive sleep apnea: absence of oronasal flow in the presence of thoracoabdominal movements for more than 10 seconds. It is considered obstructive if there is respiratory effort during apnea. Hypopnea: reduction of airflow by at least 30% of pre-event baseline and drop in thoracoabdominal movements compared to baseline for a period of more than 10 seconds, associated with a SpO2 drop of ≥4%.
Central sleep apnea: absence of oronasal flow and thoracoabdominal movements for more than 10 seconds. It is considered central if there is no respiratory effort. Spontaneous arousal: appearance of alpha rhythm between 3 and 15 seconds. The apnea-hypopnea index (AHI) was determined by dividing the total number of respiratory events by the total sleep time in hours.
Kushida et al (19), according to the AASM clinical guidelines 19,20, classify CPAP titration as:
a) An optimal titration reduces RDI <5 for at least a 15-min duration and should include supine REM sleep at the selected pressure that is not continually interrupted by spontaneous arousals or awakenings. A good titration reduces RDI ≤10 or by 50% if the baseline RDI <15 and should include supine REM sleep that is not continually interrupted by spontaneous arousals or awakenings at the selected pressure. An adequate titration does not reduce the RDI ≤10 but reduces the RDI by 75% from baseline (especially in severe OSA patients), or one in which the titration grading criteria for optimal or good are met with the exception that supine REM sleep did not occur at the selected pressure. An unacceptable titration is one that does not meet any one of the above grades. (19, p157-158)
Data were collected from the sleep questionnaire usually used at the Sleep Clinic of the Fundación Santa Fe de Bogotá (Annex 1) and the SNPSG results from the files of the sleep laboratory of the Neurology Department of the HUFSFB. Each patient was assessed according to 24 variables categorized into six groups: demographic factors, subjective indicators of sleep quality, sleep characteristics, sleep breathing pattern, diagnostic characteristics of the study, and characteristics of the CPAP titration procedure.
Categorical variables were analyzed in terms of frequency and percentage. Mean and standard deviation (SD) were used for quantitative variables of normal distribution, while median and interquartile range were used for non-normal distribution variables. In order to know this distribution, a Kolmogorov-Smirnov normality test was carried out. McNemar's test was applied and two paired sample categories were established before and after CPAP to measure AHI.
Subjects were categorized by sex, age group, body mass index (BMI), and severity of OSAHS. The latter was classified according to the AASM criteria 20: 1) non-OSAHS = AHI<5; 2) mild OSAHS = AH 5-15; 3) moderate OSAHS = AH 15-30; and 4) severe OSAHS = AH >30. This was made possible by tabulating the data in Microsoft Excel 2010 and the statistical software SPSS version 21.
During baseline and CPAP titration recording, conditions such as whether the patient remained in supine position, whether REM sleep occurred, and whether the patient remained in supine position during REM sleep were considered. Based on these variables, the baseline record was defined as an accurate diagnosis ifAH>20/h or ifAH 5-20/h when the patient remained in supine position and REM sleep was present. An incorrect diagnosis was defined if the criteria mentioned above were not met.
Results
A total of 221 patients, 161 men (72.9%) and 60 (27.1%) women, were analyzed and SNPSG studies were conducted based on the selection criteria described above. The mean age in years was 57.29 (SD=12.03) and the median BMI was 27.55 kg/m2 (IQR=5.51). In total, 208 (94.1%) SNPSG studies compatible with OSAHS were obtained, of which the majority (54.7%) hadAH>30 with an average O2 saturation of73%. Average sleep efficiency was 77.7%. Table 1 presents the demographic information.
Table 1 Demographic and clinical characteristics of the patients included in the study.
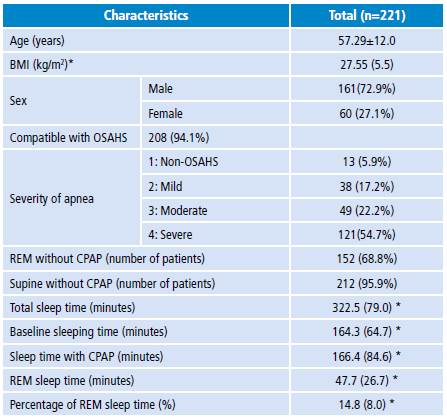
BMI: body mass index; OSAHS: obstructive sleep apnea-hypopnea syndrome; REM: rapid eye movements; CPAP: percentage of continuous positive airway pressure.
* Median and interquartile range.
Source: Own elaboration.
Patients were classified into four subgroups according to the severity of apnea. Each subgroup was analyzed for differences according to sex, finding that 96% (n=154) of the men and 90% (n=54) of the women had studies compatible with OSAHS of mild, moderate or severe AHI, and that this difference between groups was statistically significant (p=0.04). Furthermore, the variables BMI, minimum saturation in apnea, minimum saturation with CPAP, presence of REM sleep and supine position during the baseline study were analyzed in each subgroup. Differences between subgroups were statistically significant (Table 2).
Table 2 Results of clinical variables according to severity of apnea in baseline phase of polysomnography.
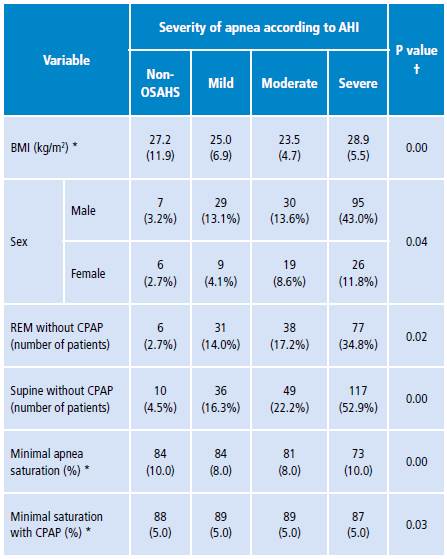
AHI: apnea-hypopnea index; BMI: body mass index; REM: rapid eye movements; CPAP: percentage of continuous positive airway pressure; OSAHS: obstructive sleep apnea-hypopnea syndrome.
* Median and interquartile range.
† Kruskal-Wallis test was used to compare medians with the severity variable and χ2 for qualitative variables.
Source: Own elaboration.
An optimal quality of CPAP titration was observed in 38.5% of the studies. When analyzing the quality of titration in relation to the severity of OSAHS, it was found that, in category 4 (severe), the quality of the titration was optimal in 24.4%, while in categories 3 and 2 the percentage of patients with optimal titration was lower, being these differences between groups statistically significant (p=0.00). A similar trend was observed in the good titration group (Table 3).
Table 3 Results of the quality of titration according to severity of apnea.
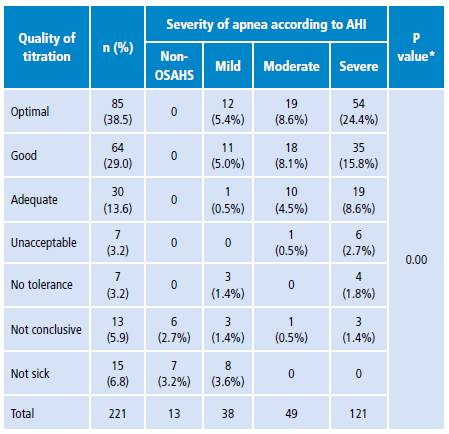
AHI: apnea-hypopnea index; OSAHS: obstructive sleep apnea-hypopnea syndrome.
* p-value for χ2 test.
Source: Own elaboration.
When comparing the severity of OSAHS during baseline recording and correction with CPAP use, it was observed that most patients in category 4 (severe) without CPAP moved to category 1 (non-OSAHS) when using CPAP (77.7%). In addition, 85.7% of patients in in category 3 (moderate) and 86.8% in category 2 (mild) converted to category 1. These differences were statistically significant according to the McNemar's test result (p=0.00) (Table 4).
Table 4 Correction of severe apnea with continuous positive airway pressure.
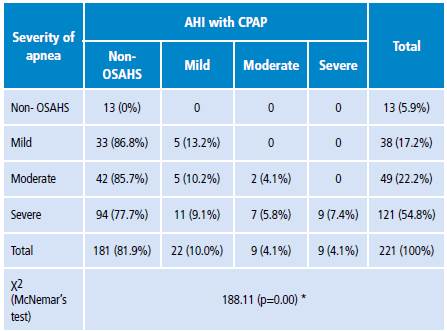
AHI: apnea-hypopnea index; OSAHS: obstructive sleep apnea-hypopnea syndrome.
* McNemar's test was used through the distribution of χ2 to determine the statistical significance of the differences.
Source: Own elaboration.
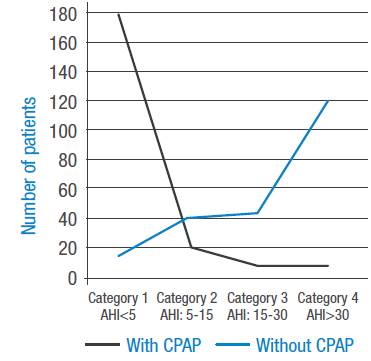
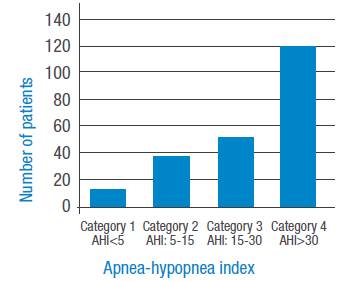
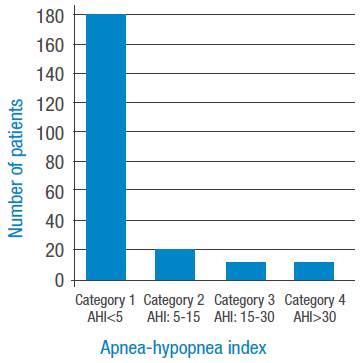
Figures 1 and 2 present the four OSAHS severity categories according to ΑΕΠ. Figure 1 shows the groups that did not use CPAP, where an increase in the number of patients with greater severity is observed. Figure 2 depicts the groups that use CPAP, making evident a marked correction ofΑΕΠ with an increase in the number of patients in category 1 (non-OSAHS) and a decrease in the other categories.
Source: Own elaboration.
Figure 1 Apnea severity index by categories without continuous positive airway pressure.
Source: Own elaboration.
Figure 2 Apnea severity index by categories with continuous positive airway pressure.
Figure 3 represents the distribution of the severity of OSAHS in the baseline studies with and without CPAP. Here, a change in the distribution of the severity of OSAHS can be observed, with predominance in the number of patients in categories 3 and 4 during the baseline study and category 1 with CPAP.
Discussion
In routine clinical practice, patients are referred by different specialists (pediatrics, internal medicine, geriatrics, neumology, otolaryngology, psychiatry and neurology) to the sleep laboratory of the HUFSFB with an indication of SNPSG due to a clinical suspicion of OSAHS. Study variables did not include data on comorbidities such as chronic obstructive pulmonary disease (COPD), high blood pressure (HBP), coronary artery disease, use of medications or application of scales of diagnostic suspicion such as the Epworth Sleepiness Scale or the Pittsburgh Sleep Quality Index, since the objective of the study was to measure the performance of SNPSG and not the association of risk factors with the diagnosis of OSAHS and its response to the use of CPAP.
In Latin America, three studies on the subject have been published in Argentina, Chile and Peru. The Argentinian study 21 included a sample of 314 patients with a diagnosis of severe OSAHS. PSG for full-night CPAP titration was performed in one group and SNPSG in the other group (216 patients), observing a rate of 73.6% adequate CPAP titration for the SNPSG group. The Chilean researchers 22 took 150 patients for SNPSG and, after focusing on the outcome of the CPAP titration, reported 80% adequate titration. A similar result was obtained in the study carried out in Peru. 23
In our sample (221 patients), an adequate diagnosis of OSAHS was obtained in 94%, while it was ruled out in 6% of cases. In the group with diagnosis of OSAHS, based on AHI, it was possible to differentiate the severity of this condition. These findings are consistent with those reported by Khawaja et al.14, Kim et al.24 and Chou et al.25, who concluded that 2-3 hours of PSG recording are sufficient to obtain an accurate diagnosis of OSAHS. Although the average sleep efficiency was 77.7% during the baseline study, it was possible to adequately identify patients with and without OSAHS.
To obtain a more accurate diagnosis of OSAHS within the first 3 hours, part of the study should be done in supine position and REM sleep should be present, since OSAHS is more prominent under these conditions. 26 Thus, 95% of patients remained part of the time in supine position and 68% had REM sleep during the baseline study. When examining these two variables by group severity, it is observed that the greater the severity of OSAHS, the greater the number of patients who partially remained in supine position and presented REM sleep. These differences had statistically significant values (Table 2). It is clear that a 3-hour baseline study in which part of the time is recorded in supine position and REM sleep is present is sufficient to obtain an accurate diagnosis of OSAHS.
This study found a higher prevalence of OSAHS in men (96%) than in women (83%). This difference is even greater in category 4 (severe OSAHS) with a statistically significant result. This finding is consistent with what has been reported in several studies. 1,5 Some SNPSG studies have also shown effectiveness in the diagnosis of upper airway resistance syndrome and its adequate titration with CPAP. 27
The quality of CPAP titration for 3 hours (166 minutes on average) ranged between optimal, good and adequate in 81% of cases (Table 1). This allows establishing an adequate treatment with the use of CPAP in a high percentage of patients and coincides with what has been reported in other studies. 21,28 In the remaining 19%, clinically adequate titration was not achieved for various reasons such as low sleep efficiency (<60%), no tolerance to CPAP, or baseline study not compatible with OSAHS. These findings suggest that a smaller percentage of patients will require a new study for CPAP titration or an alternative therapy for the treatment of OSAHS such as anti-snoring pillow (PosiForm®) 26,29 or sleep position trainer (SPT). 29,30
CPAP tolerability was high in this study (89.1%) compared to the figures reported in other studies (46-80%). 24,31,32 This higher percentage may be the result of the coexistence of other factors that influence CPAP tolerance such as the type of mask, habituation to CPAP use before starting the study, use of CPAP at low pressures at the beginning of the study, and the intervention of technicians before and during the routine study. Although the use of SNPSG may alter mask tolerability due to short titration time with respect to two-night studies, studies by BaHammam et al.28, Collen et al.31 and Sanders et al.33 showed no significant differences in CPAP use between SNPSG and full CPAP titration studies.
The limitations of this work include its retrospective nature and the fact that the analyzed sample consisted of patients referred with a clinical suspicion of OSAHS, which increases the possibility of pre-test diagnosis. In addition, the analysis did not include the arousal index during the diagnostic phase and with CPAP use, which could show changes in the continuity of sleep.
Conclusions
In this study, OSAHS diagnosis was achieved within the first 3 hours in a high percentage of the patients (94.1%) and its severity could be classified, which allowed evaluating the need for CPAP. Correction of AHI was adequate in 78% of patients with CPAP, showing the efficacy of the split-night study for diagnosis and treatment with CPAP.
The use of SNPSG reduces the waiting time for conducting the diagnostic PSG and especially the waiting time for CPAP titration. This makes easier delivering the service and is more comfortable for patients. The possibility that, for several reasons, some patients may not attend the second study for CPAP titration should be considered, because this could further delay the initiation of the treatment. This possibility decreases significantly with the use of SNPSG.
In light of this, the use of SNPSG is recommended as the first option in patients with high suspicion of OSAHS, although local cost-effectiveness studies are needed to determine whether this is a better strategy than the conventional two-night approach.