Introduction
The World Health Organization classified livestock farming as one of the anthropic activities included in the seven professional groups that are highly exposed to diverse zoonotic agents, given the required management of bovine cattle and its by-products. 1 Bovines are reservoirs of zoonotic agents for humans 2, considering the various types of infections these animals may develop 3-5 and their exposure to the wildlife that surrounds and integrates cattle systems. 4 The microbial agents with zoonotic potential are mainly bacteria, followed in order of frequency by viruses, prions, protozoa, fungi, and helminths. 6 The significant impact on the epidemiology of certain infectious diseases with zoonotic features may lead to a multi-factorial phenomenon, mostly related to anthropogenic stress such as urbanization marked by agricultural intensification, together with socioeconomic changes, ecological fragmentation, and the ensuing increased contact between animals and humans 7.
The diverse climate areas in Colombia facilitate the traditional utilization of various beef, dairy and dual purpose cattle breeds. Therefore, cattle farming is highly relevant for the country's socioeconomic development, and the department of Antioquia has the highest percentage of the total cattle inventory of the country. 8
It is well known that the impact of public policies on zoonoses is still limited in our country. 9 Acute febrile illness is one of the most common reasons for medical consultation in tropical areas, both in emergency and outpatient services. 10 In addition, when diagnosing a patient with an infectious process, the possibility of it being a zoonotic agent is often omitted and not considered in the differential clinical diagnosis. Such omission is, in most cases, related with overlapping non-specific clinical profiles that resemble more common diseases in the area, such as malaria 11 and dengue 12, which generates diagnostic confusion as well as inadequate patient management.
Furthermore, this lack of clinical suspicion regarding zoonotic diseases (particularly tick-borne diseases) directly relates to the scarce availability of diagnostic tests locally and validated for the specific detection of this type of zoonotic agents. 13 There is also previous evidence of exposure to various zoonotic agents related with livestock activities 14-16 or located in recognized Colombian livestock areas, where acute undifferentiated febrile illnesses in adults are frequent and etiological diagnosis is challenging. 17
Bearing in mind the outstanding role of the northern sub-region in the livestock sector in Antioquia, this study aims at determining the frequency of seropositivity (IgG antibodies) for certain zoonotic agents. This could be relevant in cases of people with occupational exposure in livestock contexts in the municipality of San Pedro de los Milagros (Antioquia, Colombia). In addition, this study allows exploring associated factors as a strategy to understand the dynamics of the exposure to this type of microorganisms in order to provide more accurate, preventive and diagnostic measures, as well as good occupational practices for this population.
Materials and methods
Study design and geographic location
A cross-sectional descriptive study was conducted in the municipality of San Pedro de los Milagros, located in the northern sub-region of Antioquia at 2468 m.a.s.l. and an average temperature of 16°C. About 80% of the production farms in the municipality are dedicated to cattle farming through a specialized milk production system that represents 15% of the regional milk production and is one of the greatest contributions to this economic sector among the municipalities in the area. 8 The sample size was estimated in 296 farmers, based on an approximate population of 1668 cattle farmers in the municipality (50% prevalence, 95% confidence, and 5% sampling error, with an additional estimate of 10% for sample correction and compensating possible information losses). A probabilistic sampling, representative of the study area, was applied sequentially until the estimated sample size was achieved.
Collection of information and blood samples
A survey containing socio-demographic, clinical and occupational information was conducted. Participation was voluntary and informed consent was obtained for epidemiological use of the data. The Bioethics Committee of the University Research Campus (Comité de Bioética de la Sede Investigación Universitaria, CBEIH-SIU) authorized this study through Registration code No. 11 -35-334, issued on February 4, 2011. Also, the ethical principles for medical research in humans were respected according to the Declaration of Helsinki (2013) 18, thus guaranteeing the ethical validity of the research and an average risk for participants. The risk of this study was classified as minimum according to Resolution 8430 of 1993. 19
On the other hand, blood samples were collected using the Vacutainer system, and then preserved and transported to the laboratory in sterile vacuum tubes without anticoagulant to undergo serum separation procedure.
Serological analyzes for the detection of IgG antibodies
Specific IgG serum antibodies were detected against Babesia bovis, Babesia bigemina, Anaplasma phagocytophilum, Ehrlichia chaffensis, Borrelia burgdorferi, Coxiella burnetii and Francisella tularensis by indirect immunofluorescence techniques (IFA-Fuller Labs, CA, USA), according to manufacturer's instructions for specimen handling (a 1/16 dilution was used in sera screening for C. burnetti and a 1/64 dilution for the other infections under study). The results were subsequently interpreted.
Each trial included a positive and a negative control. A sample was considered positive if bright, well-defined elementary bodies and fluorescent green apple coloration were observed; reactivity for Phases I and II of C. burnetti antigens was considered in the results related to the bacterium. The expected sensitivity and specificity of this test according to the manufacturer's report is 98 and 100%, respectively.
A rapid agglutination test was performed (Brucellosis Antigen Rose Bengal®, IDEXX Laboratories, Inc., France) to detect serum IgG antibodies specific for Brucella abortus and Brucella suis, according to the manufacturer's instructions. The sample that revealed agglutination (thin and uniform) was considered as a positive and the absence of agglutination as a negative result. A solid-phase immunochromatographic assay was performed for the differential and qualitative detection of IgG and IgM antibodies against Leptospira interrogans (SD BIOLINE Leptospira®, Standard Diagnostics, Inc., Alere is now Abbott, USA), following the manufacturer's instructions. The sensitivity expected and reported by the manufacturer was 97.7% and the specificity was 95%. An ELISA test was performed to detect IgG antibodies specific for Toxoplasma gondii (Toxo®, HUMAN Diagnostics Worldwide, Germany) according to the manufacturer's instructions.
A spectrophotometer at 450 nm, with a reference filter of 630 nm, was used for reading. The results were expressed in an index according to the optical densities (OD) of the cut-off control sera: average OD values of the sample/2. The expected sensitivity of the test was 100% and the specificity, 97.4%. Each test included negative and positive controls.
Statistical analysis
The study group was described by calculating proportions and summary measures. The frequency of seropositivity calculated for each of the microorganisms studied was compared with demographic aspects through prevalence ratios with 95% confidence intervals, binary logistic regressions, and Pearson's Chi-square and Fisher's exact tests. Significance levels of 0.05 were considered for all analyses; the SPSS Statistics for Windows, Version 21.0 was used for this purpose. 20
Results
The participants of the study were 328 farmers, 13% female and 87% male. The ages ranged from 19 to 76, with an average of 48. Regarding central values, 50% were between 48 and 57 years old and the age of most participants ranged between 46 and 69 years. For both sexes, the lowest ratio was made up of individuals under the age of 35. Most participants had at least completed elementary education, 96.6% had worked for more than 10 years in cattle farms, and 7.6% stated that they had been bitten by a tick at least once (Table 1).
Table 1 Characterization of some demographic conditions of the population of study.
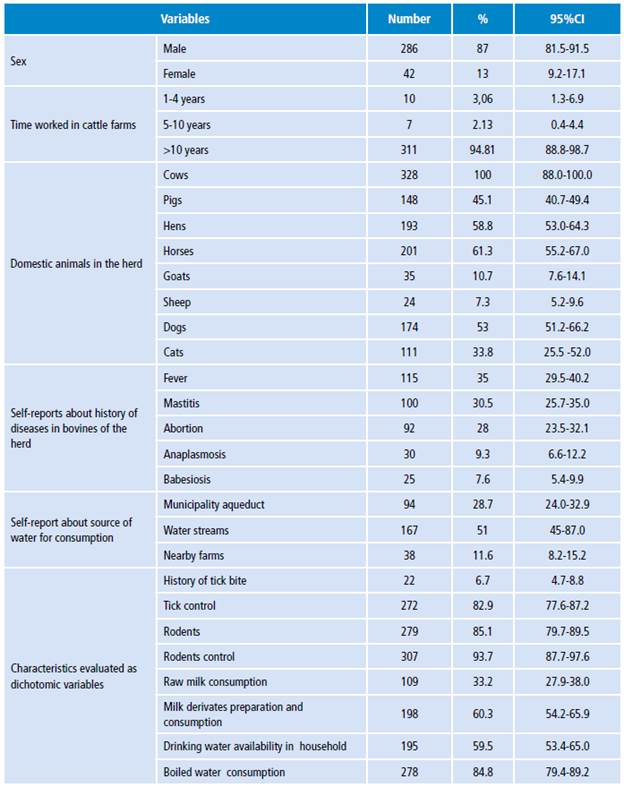
Source: Own elaboration.
Regarding seropositivity for IgG antibodies, 271 (82.6%) samples were positive for any of the evaluated tests. The overall frequency found in the population for anti-Ζ gondii IgG antibodies was 47.6% (n= 156); 33.5% (n=110) forB. burgdorferi; 13%(n=42)for E. chaffensis; 6.1% (n=20) showed Phases I and II IgG antibodies for C. burnetii; 5.8% (n=19) for A. phagocytophilum; 5.2% (n=17) for ,Ë tularensis; 1% (n=3) anti-IgG; and 0.6% (n=2) IgM fori, interrogans. There were no seropositive results for either!?, abortus, B. suis, Β. bovis or B. bigemina.
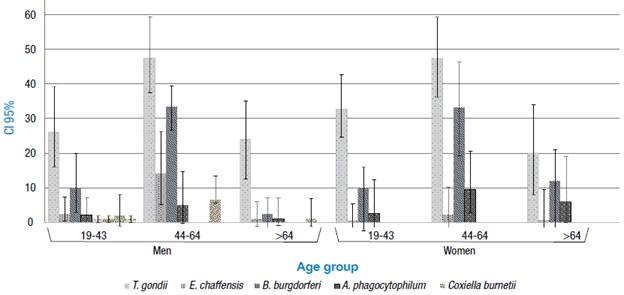
The frequency of seropositivity was statistically higher as the age group increased: 0.7% in individuals under 30 years of age and 40% in older adults. The frequency of T. gondii (47.3%), B. burgdorferi (33.3%), A. phagocytophilum (9.52%), E. chaffensis (2.3%) andL. interrogans (2.3%) was statistically higher in women over 50; the frequency of C. burnetii (2.3%) and F. tularensis (2.3%) was statistically lower in young adults. On the other hand, the seropositivity of T. gondii (47.5%), B. burgdorferi (33.5%), E. chaffensis (14.3), C. burnetii (6.64%), F. tularensis (6%), A. phagocytophilum (4.89%) andL. interrogans (1.4%) was statistically higher in men between 45 and 50 years of age (Figure 1).
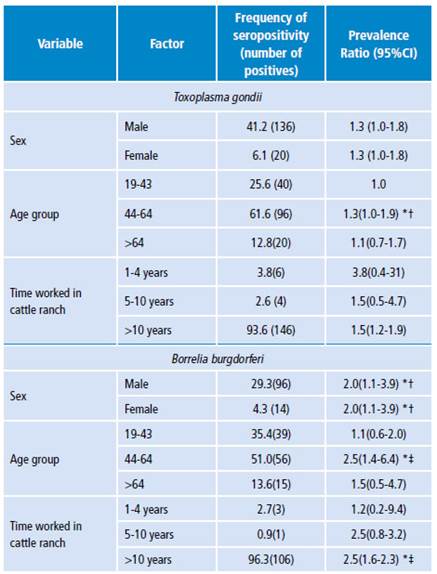
The frequency of both T. gondii and B. burgdorferi was statistically associated with sex, age group and time worked in cattle ranches, being higher in men than in women between 44 and 64 years of age with 30% (prevalence ratio 1.30) and 20% (prevalence ratio 2.0) respectively, and 50% (prevalence ratio 1.50 and 2.50 respectively) in farmers with more than 10 years of work experience (Table 2). Regarding the other factors analyzed, no statistical correlation was observed with the seropositivity detected in this study.
Discussion
In general, serological evidence of exposure to the zoonotic microorganisms studied here has been scarcely studied among cattle farmers in Colombia.
In this case, the frequency of seropositivity of anti-T. gondii IgG antibodies detected in the study population (47.6%) coincides with findings of various research works carried out in the country that have suggested that the prevalence in the general population of Colombia is approximately 47%, which also increases with age and varies significantly between regions. 21 Previous reports found a similar frequency; for example, in a study conducted in China, 40% of farmers showed IgG antibody titers. 22
Serologic evidence indicates that toxoplasmosis is one of the most common human infections throughout the world. 23 In this study, sex, an older age, and the time worked with livestock herds proved to be related with seropositivity for IgG antibodies against the infections analyzed, more in men than in women, which may be strongly associated with the ratio of men to women studied (higher than 6:1; 136 against 20, respectively). Similar findings have been reported in populations dedicated to livestock farming since the frequency of T. gondii antibodies (39.41 and 35.96% by ELISA and IFAT, respectively) increases with age, exposure to different animals and type of farming management and purpose. 24,25
It is important to consider that humans can become infected in multiple ways, which are not exclusive to a livestock context or to occupational history of exposure in people dedicated to livestock. Some infections may occur by ingesting undercooked meat from animals harboring tissue cysts (for example, cattle, goats, sheep and pigs), by consuming food or water contaminated with cat feces, by environmental samples (such as fecal-contaminated soil or litter when cleaning cat litter boxes), and by blood transfusions, organ transplantations or transplacentally from mother to fetus. 23
Comparable results were obtained regarding B. burgdorferi, whose resulting prevalence in this study was 47.5%. The highest prevalence was observed in individuals aged between 44 and 46 years (51%). Such results are higher than those reported by Miranda et al.26, who obtained a frequency of 23.3% in agricultural workers and which was higher in subjects between 41 and 50 (33.3%), followed by 30% in subjects between 31 and 40. The differences between these results may be influenced by the diagnostic performance of the different techniques employed in each study. In this study, only the IFA technique was used with an exposure screening plan; however, this test is hindered by the interference of cross-reactions between different microorganisms. Accordingly, the frequencies reported here are expected to be lower when using additional Western blot tests, since such tests are considered confirmatory for this type of infection. It is important to highlight the scarce information available on the exposure to this species in the local context. Therefore, these results are valuable as an initial epidemiological baseline.
With reference to E. chaffensis, the seropositivity obtained in this study was 13%. In our country, ehrlichiosis has been barely studied. However, such results are consistent with reports by Ripoll et al.27 who, after the application of IFA techniques, reported a prevalence of 14% in healthy subjects from an Argentinian population. This could suggest a potentially homogeneous distribution of this zoonotic agent in South America, which may be explained by similar climatic and agroecological conditions in the region.
Regarding A. phagocytophilum, there are currently few studies in Colombia. The frequency found in our study was 5.8%. These results are inconsistent with reports by Máttar & Parra 28, who reported the first evidence of A. phagocytophilum in the department of Córdoba in 81 workers evaluated, with seroprevalence of 20%. (28) Nevertheless, the results of a study conducted by Jaimes-Duenez et al.29, using molecular methods, showed a prevalence of 59.3% (n=275) for Anaplasma spp. in cattle in the municipalities of Necoclí and Turbo in the department of Antioquia, which proves the circulation of these microorganisms in productive livestock systems. In the United States, the results of a study conducted by Aguero-Rosenfeld et al.30 showed a prevalence of 11.3% for A. phagocytophilum. Despite the different study populations, the evidence of infection found is relevant as research about this type of population with occupational exposure is scarce.
A recent study suggested the circulation of these bacteria in sheep and goats from some herds in Colombia 31 and it highlights the possibility of occurrence of infections in people linked to livestock handling. In the present study, the frequency for C. burnetii was 6.1%, and such figure differs from previous reports about workers in cattle farms of Antioquia 32 and rural workers from Córdoba, in which the researchers found a prevalence of 14.7% and 26.6%, respectively. 28 These frequencies are even lower than the reports in other countries, such as Holland, which resulted in a prevalence of 83.8% in studies with populations at risk. 33,34 In our field, however, the actual prevalence of this type of infection is still unknown and its role as a causing agent of disease in humans may be underreported. Even if an association with the evaluated factors was not found in our study, the population evaluated had direct contact with the animal, which may categorize such individuals as an occupational risk group.
In general, it is important to emphasize that the MAT test, and/ or ELISA, are considered the tests of choice for the study of L. interrogans. The frequency of seropositivity for this species in our study was 0.6%, which is low in comparison with the previous results of Najera et al.35 in Córdoba and Rios et al.36 in Sucre in Colombia, who reported a frequency of 13.1% and 13.3%, respectively. These results may be explained by the use of IgG and IgM ELISA techniques, whose sensibility is higher than the solid-phase immunochromatographic assay used in this study. In this sense, it is interesting to consider that Swapna et al.37, in India, employed three methods: IgG and IgM ELISA, immunochromatographic tests and MAT; these researchers compared the sensitivity and specificity of these three methods and proved that MAT had higher sensitivity than IgG and IgM ELISA when comparing only those techniques. Conversely, when IgG and IgM ELISA and immunochromatographic tests were compared with MAT, ELISA showed higher sensitivity and specificity. However, the method used in this research detected IgM antibodies, suggesting recent infections and evidencing the possible contact of the microorganism with the population. There are several reservoirs described for Leptospira spp. (among them rodents, canines, swine, bovines, equines, goats, rabbits, and bats); rodents and bovines are considered predominantly important, because the pH of their urine is alkaline, and this favors the survival of the bacteria. This epidemiologic aspect justifies its study and attention in livestock systems. 38
These results suggest the high degree of susceptibility and vulnerability to which the reference population is exposed. Regarding occupation, the risk is considered to increase in response to the duration of the activity, which may lead to more contact with animals. Pathogens studied make part of the etiology of emerging infectious diseases, with a predominance of zoonoses (60.3%), out of which 72% originates in wildlife. Some of these are transmitted through vectors that develop favorably in tropical environments and that contribute to 23% of the emerging infectious diseases burden. 7
Our field faces deficient healthcare, few hospitals or entities that provide access to laboratory tests to establish a differential diagnosis, and the possibility of underreporting. The results presented here are coherent with others regarding lack of surveillance and diagnostics for zoonotic agents in human clinics 39, which limits clinical suspicion of this kind of infectious diseases. In this sense, there is a need to revise and implement more complete strategies for acute febrile illness treatment algorithms 17 and educate medical and veterinary staff about these zoonotic pathogens.
Conclusions
This was the first study conducted in the Northern sub-region of Antioquia that considered serological evidence of exposure to seven zoonotic pathogens. This research allowed proving that there is significant seropositivity for T. gondii, B. burgdorferi, E. chaffensis, C. burnetii, A. phagocytophilum, F. tularensis, and L. interrogans. With this in mind, this research, conducted in the Colombian Andean region, provides further insight into the study of tropical zoonotic and vector-transmitted diseases.
Hence, there is a need of conducting ongoing studies of this kind and of further interdisciplinary work aimed at analyzing zoonotic diseases holistically, in such a way that the diverse factors involved in their emergence or reemergence are studied. To get better knowledge about these zoonotic agents and their transmission dynamics in local and national livestock contexts, future epidemiological studies and preventive measures should also be taken.