Introduction
Papillary thyroid carcinoma (PTC) is the most common thyroid neoplasm. The incidence of thyroid cancer is estimated at 3.1 per 100 000 inhabitants worldwide; in Colombia, the estimated incidence in 2008 for women was 17.1 per 100 000 inhabitants, and for men 0.8 per 100 000 inhabitants. 1 Between 2003 and 2007, the Instituto Nacional de Cancerología Empresa Social del Estado (National Institute of Cancerology) reported the frequency of thyroid carcinomas by histopathological type: papillary (88%), follicular (3%), medullary (3%), Hürthle cell (1%), anaplastic (1%) and other or unclassified (4%). 2
There are various risk factors associated with the development of PTC, including radiation exposure, iodine deficiency, hormonal factors, and genetic factors. 3 More than 85% of patients achieve 10-year survival, while 10-15% follow a more aggressive clinical course that usually begins with a positive response to therapy and then, for poorly understood reasons, does not respond to radioactive iodine, leading even to death. 4
The prognostic factors identified include classification scores such as the TNM staging system (spread of cancer to other parts of the body); other factors such as age ≥45 and male gender are worse prognosis indicators. 5 Also, family history and perioperative levels of thyroid stimulating hormone (TSH) have been linked to more advanced stages with extrathyroid extension and metastasis to lymph nodes. 6
Molecular markers such as the V600E mutation in the BRAF gene, specific mutations in the RAS gene, and rearrangements or fusion of the PAX/PPARG and RET/PTC genes have been reported in thyroid cancer cases. 7 Prevalence rates between 2.5% and 44% have been established depending on the geographical region: they are high in children from Belarus and Ukraine who developed PTC after the Chernobyl catastrophe 8, but are low in cases where RET/CPT have been reported in areas with sufficient iodine intake, such as Japan 9; rearrangement is predominantly found in the papillary variant of thyroid carcinoma. 10 The RET proto-oncogene encodes a tyrosine kinase transmembrane receptor involved in the transduction of various signals related to cell transformation. 11 To date, at least 26 RET/PTC rearrangements have been described, although the presence of RET/PTC1 and RET/PTC3 rearrangements is greater. 12 RET/PTC is more common in classical tumors and in microcarcinomas, but the follicular and solid variants are rare, thus becoming an aggressive PTC subtype associated with RET/PTC3; the classical variant is associated with RET/PTC1. 13
The prognostic value of the RET/PTC rearrangement is unknown. Some studies have associated it with a higher percentage of relapse, lymph node metastasis and extrathyroid extension; some have documented a low proliferative activity with decreased cytoplasmic expression of E-cadherin; others have not detected any correlation between clinical-pathologic characteristics and RET/PTC. 14-16
Chronic lymphocytic thyroiditis is an autoimmune disorder that has been associated with papillary thyroid cancer; however, such association has not been fully established. Some authors state that it leads to better prognosis 17, while others relate it to more invasive tumor features. 18 Its association with the presence of RET/PTC is even more uncertain; in this regard, Sheils et al.19 found suggestive findings that RET/PTC1 rearrangement in patients with Hashimoto's thyroiditis is an early event of malignant transformation.
The objectives of this study were to describe the presence of the RET/PTC rearrangement in a population of Colombian patients with papillary thyroid cancer, and to evaluate the presence of lymphocytic thyroiditis in the samples by describing its relationship with demographic, clinical and histopathological characteristics.
Materials and methods
Design, population and execution
This descriptive study included papillary thyroid cancer patients operated between January 2013 and July 2015 at the Hospital de San José in Bogotá D.C., Colombia. The clinical records of patients who were taken to thyroidectomy with a diagnosis of papillary thyroid cancer were reviewed, and then histopathological characteristics were determined (histological type; tumor size; thyroiditis; lymphatic, vascular and nodal involvement; tumor extension, multicentricity and bilaterality); patients with and without lymphocytic thyroiditis were selected. Finally, the quality of paraffin tissue samples was verified for molecular testing. In addition, the variables age, sex, comorbidities, findings of post-therapy remnants, pre-ablation thyroglobulin, antithyroglobulin antibodies and relapse after iodotherapy were included in the records.
Tumor specimen processing and genetic analysis
Tumor tissue was studied by a pathologist using light microscopy to demarcate the tumor. From the paraffin bloc, a sample was cut into a 7 μπι-thick slice and then microdissected with a needle to ensure more than 80% of tumor tissue in the analyzed sample. The sample was collected in 1.5mL sterile tubes free of DNA and RNase. A specific paraffin-embedded tissue kit was used for RNA extraction (RecoverAll™ Total Nucleic Acid Isolation Kit for FFPE Tissues Ambion®). RNA was quantified using the Qubit RNA HS assay kit from Life Technologies®.
In addition, a reverse transcriptase polymerase chain reaction (PCR) was performed to obtain cDNA, which was used for real-time PCR to detect the RET/PTC1 gene rearrangement using an expression assay with Taqman probes (Applied Biosystems®).
DNA designed according to the RET/PTC1 rearrangement (NM_001145262.1) and cloned into a plasmid was used as positive control. The GAPDH gene and internal PCR control (IPC) were used as amplification control. The samples were prepared with a total volume of 20ul with the concentrations suggested by the qPCR assay and the amplification conditions used were: 50°Cx2min and 95°Cx10min, followed by 40 cycles of 95°Cx15sec and 60°Cx1min. Samples that adequately amplified controls and did not amplify RET/PTC1 were considered negative.
Analysis plan
The variables were entered in a Microsoft Excel 2011 spreadsheet and analyzed in Stata 13 (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP).
A descriptive analysis was carried out using absolute and relative frequencies for the qualitative variables, and measures of central tendency and dispersion according to the distribution of the data for the quantitative variables. Finally, an exploratory stratified analysis on the presence of the RET/PTC1 gene rearrangement, lymphocytic thyroiditis and prognostic variables was also made.
Ethical considerations
The study was approved by the Research Committee of the Faculty of Medicine and the Human Research Ethics Committee of the Hospital de San José-FUCS through Minutes No. CEISH 036-2015 of March 31, 2015. This work was developed following the ethical principles of the Declaration of Helsinki 20, and was considered a risk-free research according to article 11 of Resolution 8430 of 1993 of the Ministry of Health of Colombia. 21 Patient confidentiality was preserved during the analysis of the clinical history and the results.
Results
General clinical-pathological characteristics
Between 2013 and 2015, 55 patients with papillary thyroid cancer were treated at San José Hospital; 92.7% were women with an average age of 45 years (σ: 14.6); in men, the average age was 59 years (σ: 18.26). None of the patients reported previous radiation exposure (Table 1).
Table 1 Clinical-pathologic characteristics of patients with papillary thyroid cancer.
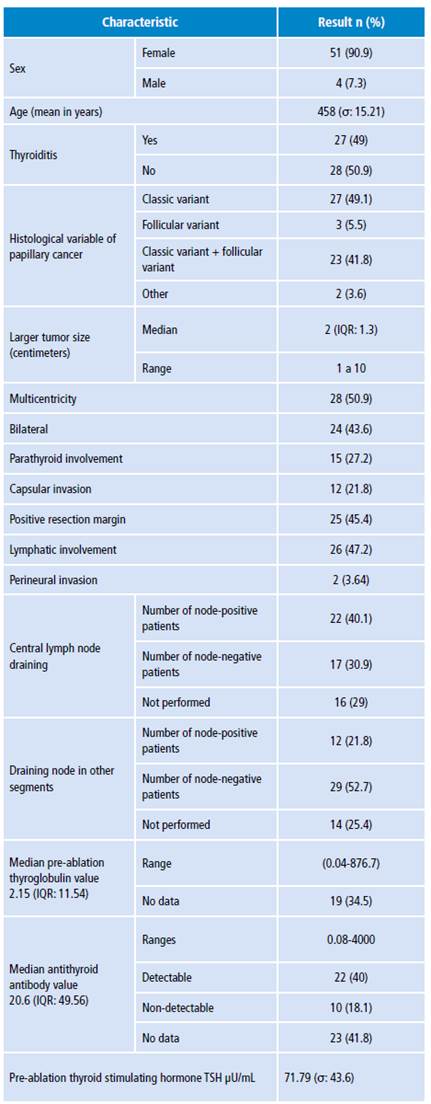
σ: standard deviation; IQR: interquartile range.
Source: Own elaboration.
The most associated comorbidities were high blood pressure (20%), hypothyroidism (15%), dyslipidemia (7.2%), diabetes mellitus (4%), gastritis (5.4%), rhinitis/sinusitis (5.4%) and migraine (4%). The most commonly found variants of papillary carcinoma were classic (49%), classic combined with follicular (41.8%), follicular (5.4%), combinations with diffuse sclerosing variant (2%), and combinations of other variants such as oncocytic and morular (2%).
The largest tumor diameter ranged from 1.1cm to 10cm. There were 22 cases (40%) with lymph node involvement in central draining (0-15 positive lymph nodes), with median of 1 and interquartile range of 3, and 12 cases (21.8%) with lymph node involvement in other segments (0-20 positive lymph nodes) and median of 0 with interquartile range of 1.
The mean pre-ablation thyroglobulin value was 34.78 ng/mL (0.0476.7 ng/mL). Distant metastases were observed in the case with a pre-ablation thyroglobulin value of 876.7 ng/mL. Antithyroglobulin antibodies averaged 218.32 IU/mL (0.08-4000 IU/mL), with detectable antibody values above 10 IU/mL in 40% of the cases (Table 1).
Of the patients analyzed, 6 (13%) relapsed and 10 (18%) did not return to follow-up, so no data are available. As for the finding of remnants in post-radiotherapy monitoring, 16 cases were negative, 19 reported remnant uptake in the thyroid bed, 1 presented uptake in other areas of the neck or mediastinum, and another had distant metastases.
Lymphocytic thyroiditis and prognostic features
Lymphocytic thyroiditis was only found in females (27 cases), and 92.3% of these patients had no family history of papillary thyroid cancer. The percentage of lymphocytic thyroiditis was 37% in the classical variant only, 55.8% in classical and follicular variant combination, and 3.7% in the classical and sclerosing variant combination.
Multicentric involvement, bilaterality, capsular invasion and positive resection margin were higher when lymphocytic thyroiditis was present, while parathyroid and lymphatic involvement and vascular invasion were lower in these patients (Table 2).
Table 2 Main characteristics related to lymphocytic thyroiditis.
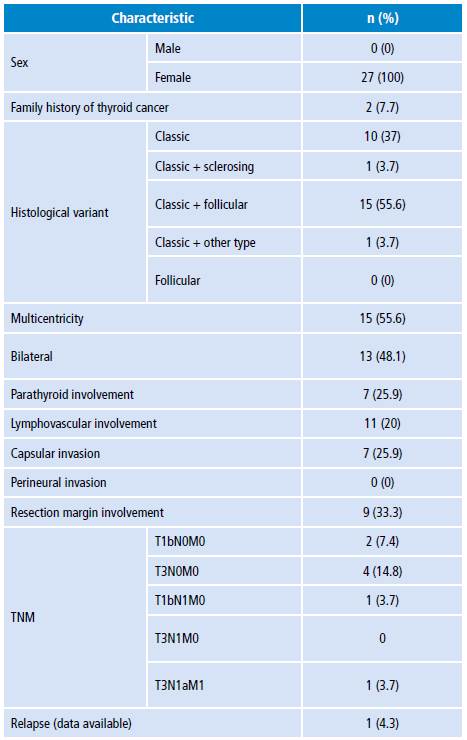
TNM: classification system (T: primary tumor; N: nearby lymph nodes; M: metastasis)
Source: Own elaboration.
In cases with lymphocytic thyroiditis, post-therapy thyroid remnant was negative in 66.7%, while it was positive in 26.7%; in addition, lung metastasis was observed in 6.7% of the cases.
The presence of lymphocytic thyroiditis, according to the TNM classification, was at stages T3N0M0 and T2N1Mx in 24.8% of cases, and at stage T1bN0Mx in 11.1% (Table 2).
The mean age of the patients with lymphocytic thyroiditis was 41.81; the median pre-ablation thyroglobulin value was 1.42 (range 0.04-876.7), which is lower compared to patients without lymphocytic thyroiditis; the median antithyroid antibody was 123.88 (range 0.08-4000), higher compared to patients without thyroiditis (Table 3).
Table 3 Description of lymphocytic thyroiditis with prognostic characteristics.
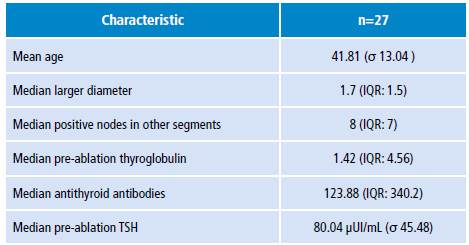
σ: standard deviation; IQR: interquartile range.
Source: Own elaboration.
A description of lymphocytic thyroiditis and prognostic characteristics was made (Table 3), finding a relationship between the presence of thyroiditis and the number of positive nodes in segments other than central draining, as well as the antithyroid antibodies value.
RET/PTC1 analysis
RET/PTC 1 rearrangement was evaluated in the RNA extracted from histological samples in 55 cases. 3 samples did not have sufficient material, so they were not analyzed; 25 samples were negative for RET/PTC1 rearrangement (amplification of controls was adequate and no amplification of RET/PTC was obtained), of which 12 came from patients with chronic lymphocytic thyroiditis; degradation of nucleic acids was found in 27 samples (cases in which neither the control nor the RET/PTC gene was amplified, but the internal control of the reaction was).
Discussion
The study found a higher percentage of PTC in the female sex; this coincides with the reports found in the literature, in which the frequency of presentation in women ranges from 60% to 83%. 18,22,23
The classic variant had the highest percentage in the present study, which agrees with another Colombian study by Sánchez et al.24 carried out in 449 cases of papillary carcinoma, 214 of which presented the classical variant. 24
This research also reported higher antithyroglobulin antibody values in patients with lymphocytic thyroiditis; these antibodies may interfere with thyroglobulin measurement, which is the primary biochemical marker used to monitor patients. 25,26
With respect to lymphocytic thyroiditis, more lymph nodes were found to be involved in segments other than central lymph node draining; however, the role of this entity and its prognostic performance has been controversial. According to Park et al.27, it does not affect the course of papillary thyroid cancer; for Girardi et al.28 it is a beneficial factor with a smaller diameter, less frequency of extrathyroid involvement and earlier clinical-pathologic staging; and according to Guzmán et al.18, it is associated with greater persistence/recurrence of the disease, although not with lymph node metastasis to lateral chains. As for lymphocytic thyroiditis and other prognostic variables, such as age, multifocality, multicentricity, and neurovascular invasion, no association was reported.
The presence of lymphocytic thyroiditis was documented in the histopathological studies of the samples where RET/PTC1 was searched; this is striking since up to 95% of RET/PTC1 positivity has been described in some series of cases with lymphocytic thyroiditis. 19
This study did not detect the RET/PTC1 rearrangement in any of the 25 samples on which the test was performed; similar works have proven a low prevalence of this rearrangement. For example, the study by Liu et al.29 detected RET/PTC1, RET/PTC2 and RET/PTC3 rearrangements in 8 of 105 samples taken from papillary thyroid carcinomas in Taiwan, with a much lower prevalence when compared to results reported in other countries using a similar methodology.
The El-Abdallah & Junaid study 30 analyzed 50 fresh tissue samples and 138 paraffin-embedded thyroid tissue samples that were taken from a Kuwaiti population; no RET/PTC1 rearrangement was found in the analyzed samples. Finally, in the study by Rao et al.22 -conducted in a population of Chennai where an approximate prevalence of 80-85% of thyroid malignancies is documented- 30 samples were analyzed to evaluate the estimation of the frequency of RET/PTC1 and RET/PTC3; to this end, total RNA was isolated and a quantitative evaluation of PCR rearrangements was made in real time, finding RET/PTC3 rearrangements in 86.6% of the cases and no case of RET/PTC1 rearrangement.
In Colombia, Ballén et al.31, who aimed to establish the prevalence of different mutations such as BRAF, K-RAS, H-ras, N-ras and the RET/PTC1, 2 and 3 rearrangement, reported that, in 31 samples, 56% of the cases were positive for BRAF and 3.7% for N-ras; no cases were detected for any of the isoforms of RET/PTC rearrangement.
Having said that, and considering the high variability described in different populations, it should not be ruled out that the prevalence of this rearrangement was low enough in the studied population so that it could not be found in the evaluated sample.
The restricted number of patients in which the RET/PTC1 rearrangement was measured is one of the limitations of the study. Another limiting aspect, from a technical point of view, that affects the expression of the RET/PTC rearrangements is that the RNA sequence analysis takes into account degradation by RNases; in this respect, the time necessary for handling and processing the tissues is highly relevant.
Furthermore, formalin, the standard fixative used in most anatomic pathology laboratories, preserves tissue relatively similar to its in vivo morphology through cytoskeleton fixation and soluble proteins. However, reversible cross-links between proteins and nucleic acids may form, as well as random breaks, resulting in highly fragmented nucleic acids. 32
In this study, the samples were taken retrospectively, which reduced the number of cases for analysis due to possible degradation of nucleic acids in paraffin samples. Nevertheless, despite these inherent limitations, this is the first work that sought to establish the presence of the RET/PTC1 rearrangement in patients with PTC in the Colombian population.
Conclusions
The presence of the RET/PTC1 rearrangement was not documented, perhaps because it had a low prevalence in the patients with papillary thyroid cancer studied here. Studies with a larger sample of patients are needed to confirm the low prevalence of the mutation and to look for other molecular markers to correlate it with prognosis.















