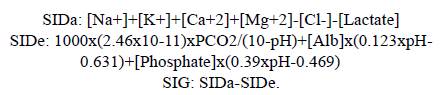Introduction
Acid-base balance (ABB) in blood has been under permanent study since Lawrence J. Henderson first presented this approach in 1908. 1,2 The definition of Arrhenius acid (substance that dissociates to form hydrogen ions) and the discovery of the law of mass action are some of the related advances. 3
Henderson's proposal arose in the context of many key chemistry breakthroughs and the birth of physicochemistry as a scientific discipline. Later, in the 1960's this approach was extended with the concept of excess base (EB), which sought to quantify the metabolic component and develop curves that correlated pCO2, HCO3 and pH; the so-called practical approximations or "thumb" standards that are used to classify acid-base disorders derive from said curves. 4-7 At present, this proposal is known as the "classical" or "traditional" approach to understanding acid-base physiology; it analyzes ABB based on different variables, the most important being bicarbonate and carbon dioxide (CO2).
In order to complement Henderson's model, in the 1970s Emmett & Narins 8 proposed the anion gap (AG), a method for electrolyte analysis that identifies possible causes of metabolic acidosis. Patients with metabolic acidosis are classified into the normal AG or high AG category, leading the clinician to suspect some specific causes of the acidosis.
The other model proposed for acid-base analysis was presented in the 1970s by Peter Stewart: the so-called physicochemical approach. It states that pH is determined by three independent variables: strong ion difference (SID), total weak non-volatile acids (ATOT) and partial pressure of carbon dioxide (pCO2). (7,9) Currently, concepts such as effective SID, strong ion gap (SIG), base excess contributed by unmeasured anions (BEua), and corrected AG have emerged as an extension of this model, and together are sometimes called "semiquantitative" approach. 6,10
One of the main advantages of the physicochemical model is its explanatory capacity. Its advocates state that it explains the causes of an acid-base disorder based on independent variables, which is not achieved with the classic model that has a more descriptive function. However, these same advocates say that the classical model may gain relevance when referring to patients with severe sepsis and septic shock, a condition in which classic metabolic acidosis is a common and complex disorder that causes multiple organ function alterations and is associated with worse clinical outcomes. 11-13
Based on Stewart's proposal, several authors have suggested to reclassify ABB disorders taking into account the independent variables, and to construct a new clinical language to this end. 14 This has generated conflicts as this approach focuses on understanding anions as acids, while protein and electrolyte disorders are considered equivalent to acid-base disorders for the construction of such language, which is controversial in the literature. 15 In this sense, normal pH, BE and pCO2 values can be reported along with some abnormal ATOT or SIG values, which can be understood as acid-base disorders in the Stewart model, but are not considered part of the acid-base sphere in the classical approach. It could also be understood from the opposite perspective: abnormal values in these variables can be interpreted as acid-base disorders without having a well-defined nature in the context of the critical patient. 16 Furthermore, Stewart's classification of acid-base disorders is debatable for several reasons: first, they are determined taking "normal serum values" as reference that are applicable to healthy individuals; second, different cut-off points are used; and finally, whether they can be applied in critical patients has not been established.
In this context, the objective of the present study was to compare the two diagnostic approaches to EBB in patients with severe sepsis hospitalized in intensive care units, and to raise a discussion focused on the classification of acid-base disorders, especially in the case of metabolic acidosis.
Materials and methods
Prospective observational study conducted at the Intensive Care Unit (ICU) for adults of the Hospital El Tunal in Bogotá D.C., Colombia. The sample was comprised of patients with severe sepsis, older than 18 years and with an ICU stay of >24 hours. Patients with chronic pulmonary pathologies, liver failure, chronic kidney failure undergoing dialysis therapy, or patients who required renal replacement therapy in the first 24 hours were excluded. Of the included patients, those who met the criteria for severe sepsis and septic shock according to the International Guidelines for Management of Sepsis were selected for analysis. 17 The study was carried out between January and June 2013 and the following data were obtained: socio-demographics, type of pathology on admission, origin of sepsis, APACHE II (Acute Physiology and Chronic Health Evaluation II) and SOFA (Sequential Organ Failure Assessment) scores on admission, days of stay at the ICU, and vital status at 28 days.
An arterial blood sample was obtained during the first 24 hours and the following variables were analyzed: arterial pH, bicarbonate, standard base excess (SBE), SID apparent (SIDa), SID effective (SIDe), SIG, AG, and corrected AG. Normal ranges of the acid-base variables were established according to references provided by local and international literature. 13,18,19
Arterial blood gases were processed in a blood gas analyzer AVL OMNITM 1-9 RADIOMETER. The same blood sample was used to measure sodium, potassium, chlorine, calcium and lactate in a Roche Cobas B 221 system using the direct selective ion method. Magnesium, phosphate and albumin were measured on a Roche/Hitachi Modular-P analyzer using a colorimetric method. The variables were calculated using the following formulas:
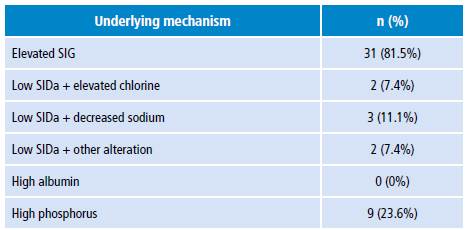
A descriptive analysis was made to estimate averages, ranges, minimum and maximum values, standard deviations and variances for quantitative variables. A statistical analysis was performed with the SPSS program, while a categorical comparison was made based on the percentage of patients according to the ABB diagnostic classification in each of the approaches. ABB was classified by both traditional and physicochemical methods, as proposed in the literature (Table 1). 14,18
Table 1 Diagnostic criteria for categorizing metabolic alteration of acid-base balance.
EB: excess base; SIDa: strong ion difference apparent; SIG: strong ion gap; P: phosphate.
Source: Own elaboration.
The study was approved by the Research Committee of the Hospital El Tunal and by the Ethics Committee of the Faculty of Medicine of the Universidad Nacional de Colombia as recorded in Minutes 167 of December 13, 2012. This work complied with the ethical considerations of the Declaration of Helsinki and Resolution 8430 of 1993 of the Colombian Ministry of Health. 20,21 Accordingly, no special informed consent was obtained because data collection and analysis of blood samples are a standard clinical practice and are covered by the hospital's general consent.
Results
Thirty-eight patients were included, of whom 21 (55%) were women. The average length of hospital stay was 8.39 days, mortality at ICU discharge and at 28 days was 21% and 24.3%, respectively, and the median of APACHE II and SOFA scores was 13 and 6, respectively. Other demographic and clinical data, as well as outcome variables, are presented in Table 2.
The results of measurements and calculations of clinical laboratory variables, ABB, electrolytes, hematological variables and renal function are shown in Table 3. The median standard BE was -6.5 mMol/L; AG, 20.11 mMol/L; and SIG, 12.04 mEq/L.
Table 3 Biochemical variables of the study population.
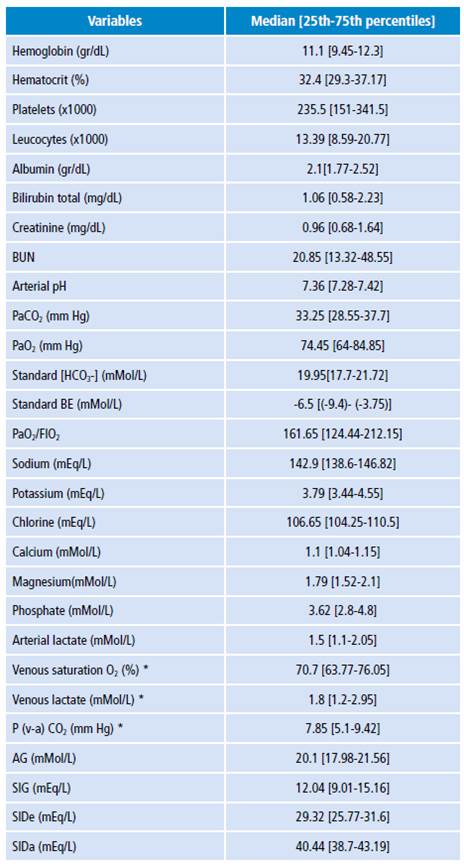
BUN: blood ureic nitrogen; EB: excess base; FIO2: fraction of inspired oxygen; AG: anion gap; SIG: strong ion gap; SIDe: strong ion difference effective; SIDa: strong ion difference apparent; P: phosphate. * Data from 18 patients (with central venous catheter).
Source: Own elaboration.
ABB disorder diagnoses based on the classification proposed in Table 1 are shown in Table 4. According to the traditional approach, metabolic acidosis was the most frequent acid-base disorder; it was found in 20 patients, while only 8 had a normal ABB. On the other hand, in relation to the physicochemical approach, all patients had ABB disorders. Metabolic acidosis plus metabolic alkalosis and hypoalbuminemia were found in all patients.
Table 4 Acid-base diagnosis according to traditional and physicochemical approach in 38 intensive care unit patients.
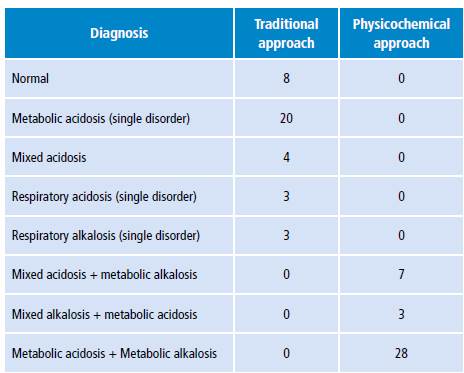
Source: Own elaboration.
Tables 5 and 6 show the different metabolic acidosis mechanisms according to the physicochemical approach. The most frequent individual mechanism was elevated SIG, which was found in 14 patients; 11 additional patients had a combination of two acidosis mechanisms; and other 11 patients had elevated SIG with some alkalosis mechanism other than albumin decrease.
Table 5 Metabolic alterations mechanisms in patients according to the physicochemical approach.
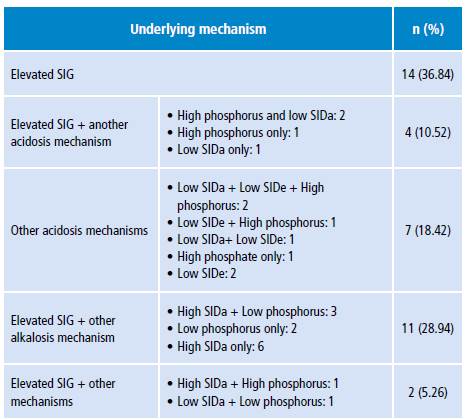
SIG: strong ion gap; SIDa: strong ion difference apparent; SIDe: strong ion difference effective.
Source: Own elaboration.
Discussion
Throughout history, acid-base disorders have been classified as respiratory or metabolic depending on the type of acid or base involved in the underlying pathological mechanism. Carbonic acid is the element involved in respiratory alterations, while the so-called organic or inorganic "fixed acids" or bicarbonate are involved in metabolic alterations. 22 The evaluation of the metabolic component is the key element of the discussion among physiological models. The traditional approach uses bicarbonate and EB as variables to assess this component, while the physicochemical approach uses SID, SIG and ATOT. 7,14
The results of the present study show disagreement in the diagnostic categorization of the acid-base disorder between the models. According to the physicochemical approach, all patients presented mixed metabolic disorders with components of both alkalosis and acidosis, while several patients had normal ABB according to the traditional approach; the most frequent alteration was metabolic acidosis and no patient presented with metabolic alkalosis.
This type of disagreement has also been described in other studies. Dubin et al.18 found that the physicochemical approach allowed diagnosing 14% more patients with acid-base alterations, most of them in the category of metabolic acidosis, which were not diagnosed by the traditional method. Mallat et al.13 reported that the physicochemical approach diagnosed 27% more patients with metabolic acidosis compared to the traditional approach. Likewise, in the study of Gunnerson et al.16, 66.7% of the patients who had normal ABB according to the traditional approach, presented some alteration according to the physicochemical approach.
In general, these studies suggest that the traditional approach may fail to identify and explain complex acid-base disorders in critically ill patients, since, according to the physicochemical approach, the metabolic acidosis resulting from an alteration in SID, ATOT or SIG and associated with the presence of hypoalbuminemia may be "concealed" in the traditional approach. 13,23,24 It has also been said that the deviation of EB and SIG from normal values is similar only when plasma buffer concentrations other than bicarbonate, such as albumin and phosphorus, are normal. 13 In this regard, it is important to note that the diagnosis of acid-base disorders does not have a universal reference standard. In this sense, two ways of diagnosing ABB are being compared and, therefore, the fact that the physicochemical approach diagnoses more patients does not necessarily mean that there are more disorders, since it may also represent overdiagnosis.
There is no doubt that strong ions and total ATOT have an impact on blood pH; however, is it appropriate to consider any alteration in SID, SIG or ATOT as an acid-base disorder? Many patients hospitalized in intensive care units have SIG alterations. For example, in the study by Antonini et al.25, 91% of the patients evaluated presented high SIG due to an increase in non-measurable anions caused by accumulations of ketones, sulphate, formate, protein dissociation products and energy metabolism intermediates, frequently observed in critical conditions; they concluded that these non-measurable anions represent the effect and not the underlying cause of the critical condition. On the other hand, Moviat et al.26 found that 62% of the critical patients evaluated presented high SIG, with higher concentrations of organic acids, amino acids and uric acid, even though they only explained 7.9% of the SIG. Finally, Gunnerson et al.16 found that of 15 patients evaluated with normal pH, pCO2 and EB, 10 presented "concealed" acid base alterations, and 7 of them had elevated SIG. Together, these results call into question whether alterations of this nature, i.e. an increase in non-measurable anions, should actually be regarded as ABB alterations.
A key point in this discussion is what is understood by acid: for Henderson the definition is the same of Arrhenius, that is, acid is any substance that increases the concentration of hydrogen ions when dissolved in a solution, while Stewart relies on the definition of Van Slyke, which leads to infer that an anion is an acid. 1,9 This discussion has been going on for many years and no consensus has been reached 27; at present, it is accepted that definitions are relevant depending on the field in which they are applied, and they are not considered more or less valid than the other from a scientific point of view. 28. In a seminal article on the subject, Siggaard-Andersen 15 widely discusses this issue and concludes that ion and protein alterations cannot be considered of acid-base nature; therefore, the categorization of a SIG alteration, for example, cannot be automatically categorized as an ABB disorder.
On the other hand, the physicochemical approach does not clearly define what metabolic acidosis is. The studies mentioned above 13,16,18 do not clearly associate diagnosis to a pH decrease, but rather imply that the alteration of a single independent variable is sufficient to categorize the patient with "metabolic acidosis". 14,18 This diagnostic categorization is described in tables that present a way of interpreting ABB; likewise, the mathematical analysis of causality proposed by Stewart leads to a potential utility in clinical practice by proposing diagnostic classifications in which independent variables that modify the concentration of H+ are equated to diagnostic categories when such variables are altered, which, as mentioned earlier, is questionable. 16
In this sense, it can be said that ABB in blood is the result of the physiological processes that occur in the body and is normal, and that the EB is the sum of the results of the metabolic processes if EB, pH and pCO2 are normal. Thus, ABB is normal and Stewart's independent variables are individual mechanisms that potentially alter pH. The question of whether an isolated disorder of one of the independent variables proposed by Stewart in the context of normal pH, pCO2 and BE should be considered as an ABB disorder is still unresolved.
Another issue related to this diagnostic categories assignment is the definition of reference values or normal values used for analysis. In the present study, the normal range was 38-42 mEq/L for SIDa, and 0-8 mEq/L for SIG, taking into account the reference values found in the literature. However, the ranges may have certain variations: Noritomi et al.23 obtained an average SID of42.45 mEq/L (±2.32) and SIG of 2.61 mEq/L (±1.64) in the control group (healthy individuals), while Gunnerson et al.16 found SIDe of 40 mEq/L (±3.8) and SIG of 1.4 mEq/L (±1.8) as normal values in healthy volunteers.
There is no evidence of studies that have established normal values for SID or SIG in healthy Colombian population. Considering reference values other than those used in this research, as is the case of other studies, may change some percentages in the results. For this reason, it is important to establish normal reference values when carrying out this type of research.
In the context of sepsis, there is no clarity about the mechanisms that cause metabolic acidosis, since aspects of the underlying pathophysiological process and the treatment put in place may be involved. Mechanisms include lactic acidosis, kidney failure, ketoacidosis, hyperchloremia, among others. 12,29 In this research the highest frequency of the "metabolic acidosis" category was caused by elevated SIG, that is, non-measurable anions according to the physicochemical approach. However, the most notable feature of the Stewart model categorization was the presence of more than one physiopathological alteration in the same patient, in whom different mechanisms of "acidosis" were identified and mechanisms of "acidosis and alkalosis" were also combined; all this is difficult to interpret in terms of their physiological meaning and temporality.
Noritomi et al.23 found that metabolic acidosis was explained by a difference in inorganic ions, reduced mainly by severe hyperchloremia and elevated SIG, while Mallat et al.13 found that 70% of patients had an increase in SIG and chlorine (most with a concomitant increase in SIG). The average SIG and SIDe values found in the latter study (28.9 mEq/L and 12.09 mEq/L, respectively) were similar to those found in this investigation. Unlike the previous ones, this study did not report a large amount of patients with low SIG and hyperchloremia; in addition, it was not possible to correlate this fact to the amount of crystalloids previously received as it was not a documented variable. Studies of this type identify the individual mechanisms of acid-base alteration by physicochemical approach; this is often regarded as an advantage of the physicochemical model over the traditional one. Nevertheless, it should be noted that identifying such mechanisms has not so far translated into specific therapeutic actions in most cases.
The nature of this study does not allow making hypotheses or novel approaches from a physiological perspective. Still, recent publications discuss the clinical approach to ABB based on traditional methods 30-32, but the physiological understanding of ABB and its alterations are far from being a completely understood subject. Researches around the topic of water dissociation as a mechanistic explanation of [H+] alteration, in orders of nanomolar magnitude 33 , mathematical models of intra- and extracellular pH regulation 34 and advances in the understanding of intra- and extracellular pH sensors 35, as well as ion management in the kidneys 36,37, are some examples of how this field advances to achieve a better physiological understanding of the topic.
This study has several limitations. First, as noted above, there was no evaluation of healthy subjects to define ranges of normality; however, the ranges used are similar to the normality values of studies done in intensive care units, and although there may be small variations depending on the population, they may not be as relevant when categorizing the patient. It was also not possible to characterize in this study the hydroelectrolytic management received before admission to the ICU, partly because many patients were referred from another institution, so the data was not obtained.
Conclusions
The physicochemical model leads to diagnose more patients with ABB disorders. Consequently, all patients had acidosis and metabolic alkalosis, and the most frequent proposed mechanism of acidosis was elevated SIG. The nature of these disorders and their clinical significance are yet to be defined.