Introduction
Childhood obesity and overweight are highly prevalent in Colombia. Currently, 1 out of every 6 children between the ages of 5 and 17 has one of these conditions,1 situation that Fernández-Juan et al.2 have reported on schoolchildren from Bogotá. Worldwide, several studies have discussed the relationship between childhood and adult lipid metabolism, and have reported that serum levels of total cholesterol, lipoproteins and body mass index (BMI) in children are predictors of cardiovascular disease (CVD) risk in adulthood.3-5
Delgadillo-Guerra & Romero-Hernández6 report that alterations in glucose and insulin metabolism associated with dyslipidemia and changes in blood pressure are risk factors for the development of cardiovascular disease (CVD) and type 2 diabetes, conditions that can occur at an early age and persist into adulthood. In addition, insulin resistance (IR) and hyperinsulinemia are risk factors for metabolic syndrome (MS) in puberty. 7,8
Considering the anabolic and mitogenic effects of insulin-like growth factor 1 (IGF-1) and its important role in development and growth, in recent years an association has been reported between this factor, as well as 3 of its binding proteins (IGFBP-1, IGFBP-2 and IGFBP-3), and the development of CVD. 9,10 It has also been suggested that the IGF/IGFBP axis may influence atherogenesis through the stimulation of vascular smooth muscle cells and foam cells proliferation, possibly by acting on lipid levels in blood and insulin sensitivity. 11
IGF-1 is an effector hormone that is synthesized in multiple organs -especially the liver-, is essential for growth, and plays an important role in mediating growth hormone (GH) function; 9,12 it is also homologous to insulin and is capable of inhibiting proteolysis and stimulating glucose uptake, glycogen synthesis and lipogenesis. 13,14 In this regard, Mohanraj et al.15 describe the relationship between obesity and circulating levels of IGFBP, while Akanji et al.11 state that alterations in the IGF/IGFBP axis may increase the risk of CVD and that increased IGF-I is associated with premature atherosclerosis and increased risk of CVD.
IGFBP-1 is one of six binding proteins that regulates IGF-1 bioactivity. Its production in the liver is regulated by insulin. 16 Its plasma concentrations have been associated with metabolic, nutritional and anthropometric factors, 17 and its levels are inversely proportional to adiposity and IR. 18 Circulating concentrations of this protein are high during fasting, malnutrition and both types of diabetes, and are low in overweight cases. 19,20 There is also evidence that suggests that IGFBP-1 is a useful predictive marker of CVD risk given its relationship with BMI, circulating proinsulin levels, MS, 21 IR11,17,22 and even fatty liver. 9,16
On the other hand, IGFBP-2 is a biomarker that allows identifying IR and high CVD risk in adults. It is the most common IGFBP expressed during childhood and the main IGFBP produced by adipocytes. Recent studies have shown an inverse correlation between the circulating levels of this protein and adiposity. A similar correlation between IR and adiposity has also been described. Determining the levels of IGFBP-2 is useful in order to deepen understanding of the correlation between nutrition, growth, obesity and CVD risk. 23-25
Regarding IGFBP-3 -protein synthesized mainly by the liver-, its circulating levels have been associated with CVD, obesity and IR. It has also been proven that it acts as an anti-inflammatory factor capable of inhibiting the pathway of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB).15
Mohanraj et al.15 reported that proteolytic IGFBP-3 is positively correlated with adiposity parameters such as waist circumference (WC), BMI, fasting insulin and IR in overweight and obese population. Moreover, several studies have described an increase in IGF-1 and IGFBP-3 levels in overweight and obese adolescents. 15,19,20 This protein appears to have an antiproliferative action in vascular smooth muscle cells, 11 which inhibits the formation of atheromatous plaque.
With all this in mind, the aim of this study was to establish the correlation between nutritional status -determined by anthropometry and serum levels of IGF-1 and its binding proteins (IGFBP-1, IGFBP-2 and IGFBP-3)- and CVD risk markers in students aged between 7 and 9 years enrolled in 2 schools.
Materials and methods
Comparative cross-sectional observational study conducted in 84 children between 7 and 9 years of age to identify the correlation between possible variations in CVD risk markers and nutritional status.
This research was carried out during the second half of 2014 in a school located in Bogotá D.C. and another in Soacha, and was approved by the Ethics Committee of the Faculty of Medicine of the Universidad Nacional de Colombia by means of Minutes No. 75 of November 28, 2013. The study complied with all ethical aspects and followed the principles of the Declaration of Helsinki; 26 it was also classified as a minimal risk research according to Resolution 8430 of 199327 of the Colombian Ministry of Health. Both children and their guardians signed an informed consent
A non-probabilistic sample for convenience was selected; students enrolled at the Instituto Educativo Distrital Llano Oriental (Bogotá D.C.) and the Liceo Integral Los Alisos (Soacha) schools were included. The selection criteria were children aged between 7 and 9 years and 11 months, with no disease that could affect the results (diabetes, systemic arterial hypertension, hypoglycemia, hyperglycemia, hypercholesterolemia and growth hormone alteration), and children who had used medications in the last month. All the children were at stage 1 on the Tanner scale (no apparent sign of sexual maturation).
Weight was measured on a Tanita scale, with the participants barefoot and dressed in light clothing; height was determined using a dry stadiometer according to the recommendations of the World Health Organization's (WHO) growth pattern instructions for Colombia.28 BMI or Quetelet index was defined as weight/height expressed in kg/m2, and WC was measured in a horizontal plane halfway between the last right rib and the iliac crest using an inelastic fiberglass tape.
Blood samples were taken from the antecubital vein by venepuncture after 12 hours of fasting and centrifuged at 3 500 rpm for 5 minutes. The sera obtained were stored at -20°C in order to measure blood glucose levels and lipid profile, and at -80°C for determining IGF-1 and its 3 binding proteins.
Total cholesterol (total-c), triglycerides (TG), high-density cholesterol (HDL-c) and blood glucose were measured using spectrophotometric enzymatic techniques; low-density cholesterol (LDL-c) was calculated using the Friedewald equation and non-HDL cholesterol (non-HDL-c) was defined as the difference between total-c and HDL-c. Serum levels of IGF-1, IGBP-1, IGFBP-2 and IGBP-3 were determined by double-binding enzyme immunoassay (ELISA) according to the manufacturer's instructions (DIAsource). This process was performed with the DS2/DSX automated equipment.
The WHO growth reference data for children and adolescents (2007) were used to interpret anthropometric variables as set forth in Resolution 2465 of 2016. 28 Moreover, weight/height, height/age and WC/age indicators were established and five subgroups with a similar number of children were formed: 1) low height-for-age and risk of low height-for-age, 2) eutrophic, 3) adequate weight and high WC, 4) overweight and 5) obese. WC in childhood was classified according to the percentiles established by McDowell et al.29 and the lipid profile fractions were classified, as indicated by Bamba, 30 according to the National Heart, Lung, and Blood Institute (NHLBI) cholesterol screening guidelines and cut-off points, 30 which are also based on the National Cholesterol Education Program study.31
The statistical analysis was performed using the SPSS program version 18.0 after creating a database in Microsoft Excel 2013. The quantitative variables were mean, standard deviation of the mean, minimum and maximum values, percentiles and count of cases on which this calculation had been made; the frequency distributions of the continuous variables were represented in bar charts, box plots and histograms. Subsequently, an inferential statistic was made and anthropometric variables and lipid profile and glycaemia were described with means and standard deviation.
Pearson's correlation coefficient was used to identify the linear relationship between two quantitative variables. Furthermore, for non-homoscedastic lipid variables, the Kruskal-Wallis test was used to find significant differences between the nutritional status groups. Likewise, an analysis of variance (ANOVA) was used to compare the means of IGF-1, IGFBP-1, IGFBP-2 and IGFBP-3 between the groups according to the nutritional status of their members. On the other hand, pairwise comparisons were applied using the Games-Howell method, which assumes different variances, in order to complement the contrasts between the groups. In addition, groups were assessed according to the nutritional status of their members using the Dunnett's test, and significant results were obtained.
Results
The average age of the children was 8.2±0.7 years; 49% attended the Liceo Integral Los Alisos school and 51% the Instituto Educativo Distrital Llano Oriental school. Table 1 shows the anthropometric variables of the participants.
Table 1 Anthropometric, lipid profile and glycemic variables per nutritional classification group.
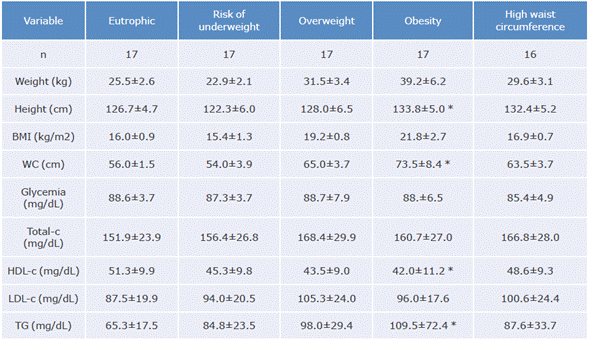
BMI: body mass index; total-c: total cholesterol; HDL-c: HDL cholesterol; LDL-c: LDL cholesterol; TG: triglycerides; WC: waist circumference.
* Significant difference (p<0.05).
Source: Own elaboration.
The lipid profile of most children were within normal values (Table 1); however, a significant number had alarming (bordering) or elevated levels compared to the NHLBI recommended limits (Figure 1). All participants had blood glucose levels within the reference values.
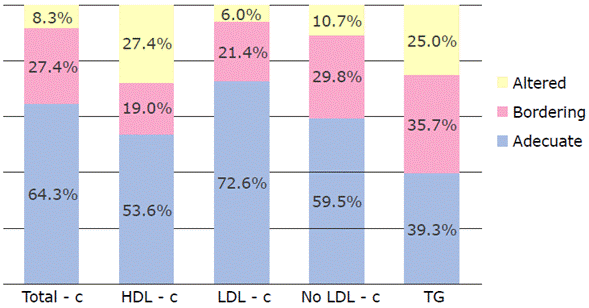
Total-c: total cholesterol; HDL-c: HDL cholesterol; LDL-c: LDL cholesterol; non-HDL-c: cholesterol no HDL; TG: triglycerides. Source: Own elaboration.
Figure 1 Percentage of children classified according to the National Heart, Lung, and Blood Institute's lipid profile cut-off points.
After analyzing anthropometric variables and the lipid profile, a significant difference was found between children in the overweight, obesity and adequate weight groups with high WC compared to children in the eutrophic and at risk of underweight groups (p<0.05).
Moreover, 62% of children with excess weight had alterations of at least one variable of the lipid profile and 26.7% were in the bordering cut-off point, which would indicate that the lipid profile values of 88.7% of the participants in this group had alterations. These results could indicate the possibility of being at risk of CVD in the long term.
Also, using the Pearson's correlation coefficient, a direct association between IGF-1 and height was observed, while the ANOVA test, Kruskal Wallis'testandthe medians allowed finding the inferences (p<0.05). The significant difference between individuals with adequate height and low height-for-age or at risk of low height-for-age was identified using the Dunnett's statistical method.
Figure 2 shows IGFBP-1 and IGFBP-2 serum levels according to the BMI classification. The lowest IGFBP-1 values were found in overweight individuals and those with a higher WC index; the lowest IGFBP-2 values were also found in overweight children.
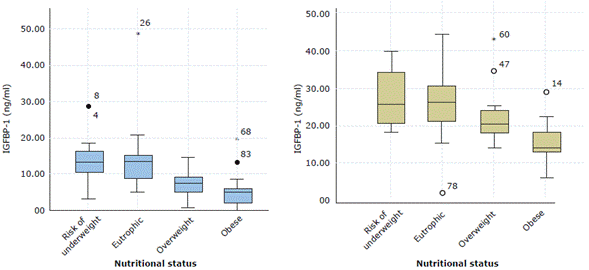
IGFBP-1: Insulin-like growth factor-binding protein 1; IGFBP-2: Insulin-like growth factor-binding protein 2 Source: Own elaboration.
Figure 2 Box plot according to nutritional status and the IGFBP-1 and IGFBP-2 variables.
With respect to IGFBP-1 levels, both the ANOVA test and the Games-Howell method established a significant difference between individuals with adequate BMI and those who are overweight or obese (p=0.000 and p<0.05, respectively). Likewise, the ANOVA analysis allowed evidencing that children with lower IGFBP-2 values were obese and overweight (p=0.001), while the Dunnett's test found that the highest statistical significance for IGFBP-2 is in the obese group.
The ANOVA analysis of the WC classification and the IGFBP-1 and IGFBP-2 levels revealed a significant difference (p=0.000), while the Games-Howell method identified that these proteins tend to decrease as the WC increases. On the other hand, IGFBP-3 did not show any significant difference according to nutritional status nor did it show any relationship with IGF-I levels.
The relationships between IGFBP-1 and IGFBP-2 levels and the lipid profile variables (Table 2) were evaluated, finding that both proteins are inversely correlated with TG concentrations and directly correlated with HDL-c values. Some variables were also compared between eutrophic and obese children (Table 3).
Table 2 Correlation coefficients between IGFBP-1 and IGFBP-2 and lipid profile variables.
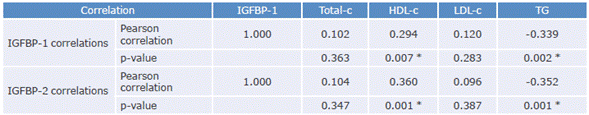
IGFBP-1: insulin-like growth factor-binding protein 1; IGFBP-2: insulin-like growth factor-binding protein 2; Total-c: total cholesterol; HDL-c: HDL cholesterol; LDL-c: LDL cholesterol; TG: triglycerides.
* Significant correlation (p<0.05).
Source: Own elaboration.
Table 3 Statistical summary of biochemical differences between eutrophic and obese children.
| Characteristics of children | IGF-1 | IGFBP-1 | IGFBP-2 | IGFBP-3 | Total-c | HDL-c | LDL-c | TG |
| Eutrophic | 244.9 | 14.4 | 266.5 | 3742.2 | 151.9 | 51.3 | 87.5 | 65.4 |
| Obese | 324.5 | 5.7 | 150.1 | 3605.1 | 160.7 | 42.5 | 96.3 | 109.5 |
| p-value | 0.035* | 0.002 * | 0.0000126 * | 0.317 | 0.161 | 0.011 * | 0.091 | 0.013 * |
IGF-1: insulin-like growth factor 1, IGFBP-1: insulin-like growth factor-binding protein 1; IGFBP-2: insulin-like growth factor-binding protein 2; IGFBP-3: insulin-like growth factor-binding protein 3; Total-c: total cholesterol; HDL-c: HDL cholesterol; LDL-c: LDL cholesterol; TG: triglycerides.
* Significant difference (p<0.05) when assessing the assumption of equality of means for the variables.
Source: Own elaboration.
In each case, the hypothesis of equality of means was evaluated (p<0.05), finding that obese individuals had significantly lower IGFBP-1, IGFBP-2 and HDL-c levels compared to eutrophic individuals. In turn, significantly lower IGF-1 and TG levels were observed in the latter.
No strong association between IGF-1 and BMI or WC was observed. Furthermore, an inverse relationship between IGFBP-1 and IGFBP-2 serum levels and BMI was identified. Finally, it was observed an increase in TG levels and a decrease in HDL-c levels in eutrophic children as weight increases leading to overweight and obesity (Figure 3).
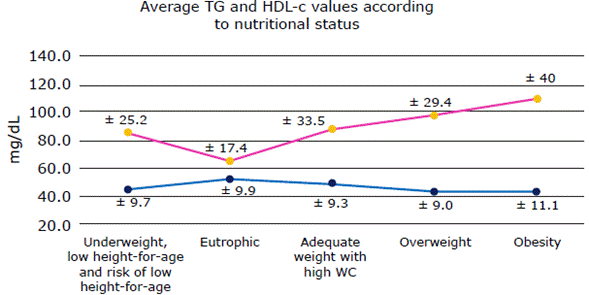
HDL-c: HDL cholesterol; TG: triglycerides; WC: waist circumference. Source: Own elaboration.
Figure 3 Mean values and standard deviation of TG and HDL-c for each nutritional status.
A linear relationship between WC and IGFBP-1 and IGFBP-2 was identified using the Pearson correlation coefficient, which was confirmed with a Games-Howell statistical test. The average level of these proteins decreases as the WC of the individuals increases.
Finally, the relationship between TG and HDL-c was analyzed using the TG/HDL-c index; 32 the latter was >2 in 32 children, of whom 72% were in the high WC, overweight and obesity groups, suggesting a high risk of IR in this population.
Discussion
The results obtained show alterations in the lipid profile, particularly high concentrations of TG and low concentrations of HDL-c, which have been previously reported33-36 and are constantly associated with future health risks. The most frequently reported risk factor for CVD is a decrease in HDL-c, which has protective functions at the endothelial level as it reduces the formation of foam cells and atheromatous plaques. Consequently, these alterations in early life are of particular concern, since they mean that individuals have a longer exposure time and, therefore, greater endothelial damage, which is considered a risk factor for IR in adolescents.
When analyzing the TG/HDL-c ratio, values >2 were observed, mostly in the overweight and obesity groups. In the study by Iwani et al.,37 this indicator was significantly correlated with the value of the homeostatic model to assess IR (HOMA-IR) and WC, while the study by Soutelo et al.32 established that it could facilitate timely decisions in health, even in children from 7 years of age.
IGF-1 was not related to BMI, but it was related to height when comparing the 5 study groups, finding lower levels in children with low height-for-age and at risk of low height-for-age compared to children with adequate height. Although IGF-1 secretion is stimulated by GH in all tissues, the response depends on nutritional status. In addition, this factor also stimulates cartilage growth; the synthesis of RNA, DNA and protein; and anabolic processes.38,39 In this sense, IGF-1 plays a key role in growth, so there is a positive correlation between its circulating concentrations and the growth rate of children.38
When comparing obese children with eutrophic children, IGF-1 levels were elevated in the first group and also had a significant difference. Mohanraj et al.15 and Park et al.40 reported similar findings. Although obese individuals have lower GH production, their circulating IGF-1 concentrations were high, which could be explained by increased pre-adipocyte expression of IGF-1 and its receptor due to the effects of insulin and cortisol; thus, IGF-1 activates the proliferation of pre-adipocytes and their differentiation into adipocytes.41
IGF-1 production in response to an increased number of adipocytes may play a role in the decreased output of GH observed in patients who suffer from obesity.20,42,43 However, this decrease in the secretion of this hormone is not associated with an alteration in the speed of growth, since obese children usually have a normal or even increased height for their age; for example, in this study, the obese group was 7cm taller than children of similar age with adequate weight.
There was no significant difference in IGFBP-3 levels according to the nutritional status; however, obese children had the lowest average IGFBP-3 compared to other nutritional statuses. This finding has been reported in other studies in which IGFBP-3 levels in obese children and adolescents were reduced42 or did not change. 42,44,45 In Switzerland, Lewitt et al.46 linked this decrease in total IGFBP-3 in obese adolescents to increased protease activity of IGFBP-3 and increased circulating fragments of IGFBP-3, and in the US, Mohanraj et al.15 reported, on the one hand, the same situation in adolescents in whom circulating levels of total IGFBP-3 were low and, on the other, the presence of proteolytic fragments of IGFBP-3 in overweight and obese subjects.
The serum IGFBP-1 level observed in the children in this study was significantly lower in the obese than in the eutrophic children. Similar findings were reported by Martínez-de Icaya et al.19 in Spain, Reinehr et al.21 in Germany, Saitoh et al.22 in Japan and Ricco et al.44 in Brazil. Specifically in Spain, Martinez-de Icaya found in a group of 7-10-year-old children that overweight children had higher serum IGF-1 levels and lower serum IGFBP-1 levels compared to controls;19 these findings in children with obesity are related to being more prone to IR.
Other relevant data reported in the present study were the relationships between IGFBP-1 and IGFBP-2 and the variables of the lipid profile. It was found that low levels of these proteins were associated with low levels of HDL-c and high levels of serum TG, which is similar to what was reported in adults by Ruan & Lai13 and Heald et al.47 These results suggest that low IGFBP-1 and IGFBP-2 levels may be associated with different factors that predispose to atherogenesis13,48 and that low serum IGFBP-1 may be an early predictor of CVD in prepubertal obesity. 13,21
Furthermore, this study found an inverse relationship between circulating IGFBP-2 levels and nutritional status. The lowest values were found in children diagnosed with obesity, which is consistent with previously reported data.23,47,49,50
IGFBP-2 has paracrine action and is the IGF-1 binding protein most expressed in the body and the main IGFBP secreted by white preadipocytes during adipogenesis.23,24 The relationship found in the present study, between decreased circulating IGFBP-2 levels and overweight and obesity may be explained by an unfavorable secretion profile of this binding protein by adipocytes, which may be associated with increased leptin and decreased adiponectin levels.49 In this regard, Hedbacker et al.51 demonstrated that leptin increases liver transcription of IGFBP2 when administered peripherally or centrally in mice with lipodystrophy.
Conclusions
The presence of high levels of cholesterol and TG in children is a serious public health problem. In the present study, alterations in the lipid profile were evidenced in overweight and obese children, so early modifications in the metabolism regarding overweight and increased adiposity are recommended.
Excess weight was positively related to TG and negatively related to HDL-c. This fact is associated with an increased risk of developing CVD, so it is necessary to promote the implementation of physical activity and nutrition education programs in schools to improve eating habits and the practice of physical activity in the child population.
In overweight children, there are alterations at the lipid and protein-binding level of insulin-like growth factors, which increase the risk of vascular disease in adulthood. Therefore, public health strategies should be established to prevent and treat excess weight in childhood, which in turn will reduce the risk of these diseases in adulthood.














