Introduction
Organ transplantation is an alternative for the treatment of patients suffering from certain chronic end-stage diseases. Immunosuppressive drugs help prevent transplant rejection, but they may have multiple side effects, including an increased risk of infection and the development of cardiovascular diseases and neoplasms. Therefore, attempts to achieve transplant tolerance since the beginning of the procedure have always been considered to reduce the harmful effects of immunosuppressors and make grafts last longer.1
The key to determining the molecular processes of the immune system is to identify how an antigen first comes into contact with it. One form of contact is the oral route, in which it has been observed that, upon recognition of the antigen, the cells of the gastrointestinal tract generate a milder immune response than if the antigen entered the body by a different route; this is known as oral tolerance.1
Bartman et al.2 have reported the success of oral tolerance in certain autoimmune diseases such as arthritis. Likewise, several authors have pointed out its efficacy in organ transplants made in murine and human models.2-21
A literature review on the induction of oral tolerance, immunological mechanisms, the regulatory cell response against exposed antigens and the experimental results of oral tolerance in organ and tissue transplants was conducted to demonstrate that this therapeutic alternative is viable in transplant patients.
Materials and methods
A literature review was conducted between February and April 2018 in PubMed, MEDLINE, LILACS and Em-base. The search was made using the following DeCS Bireme terms: "linfocitos T reguladores", "autoinmunidad", "inmunosupresión", "sistema inmune" and "tolerancia inmunológica", and the MeSH terms "T-Lymphocytes, Regulatory", "Autoimmunity", Immunosuppression", "Immune system" and "Immune Tolerance". Boolean operators were not used to conduct the search. The review included original research articles, case-control studies, and narrative and systematic reviews in humans and animals, published in Spanish and English, and with no publication time limit.
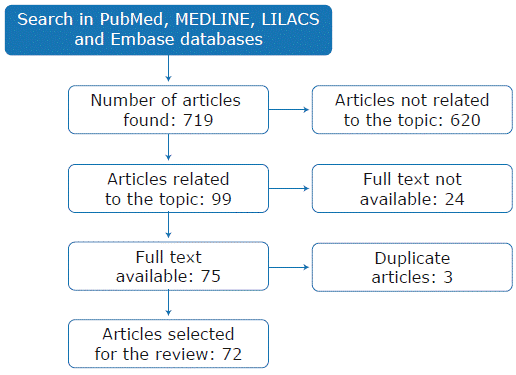
The search yielded 719 results; two co-authors simultaneously and independently reviewed abstracts and titles to determine if they met the inclusion criteria (studies on oral tolerance and human transplantation). In case of disagreement, a third author resolved the discrepancy. After this review, it was determined that 99 articles met the inclusion criteria; 24 of them were excluded because the full text was not available and 3 because they were duplicated. In total, 72 relevant articles were selected for this review (Figure 1).
Results
Of the studies selected for analysis, 52 (72%) were published between 2005 and 2018 and the country with the largest number of publications was the United States with 39 (54%), followed by Japan with 8 (11%). The predominant language was English (65 articles) and most publications were review articles (56%) and case-control studies (31%) (Table 1).
Table 1 Methodology and findings of the selected studies.
| Study/Year | Type of article | Findings |
| Bartman et al.2 2015 | Review | Commensal microbes can modify the immune response to organ transplantation locally (in the gastrointestinal tract) and throughout the organism. For example, T cell response to viral infections in the bone marrow varies depending on the commensal microbiota. The cell wall molecules from commensal microorganisms enter the circulation and modify the expression of neutrophils or their precursors in the bone marrow. It seems that transplant rejection may be influenced by microbiota in the immune system. |
| Faria & Weiner3 2005 | Review | Epithelial cells and gastrointestinal flora modify the function of dendritic cells, which induce the production of Treg cells, responsible for regulating the autoimmune and inflammatory response. Although there are some satisfactory results in animal and human models, their clinical application has not been possible. |
| Hershberg & Mayer4 2000 | Review | Intestinal epithelial cells function as APCs. They also have antigen receptors that can generate the internalization of antigens through endocytosis. Unlike the M cells in Peyer's patches, the glycocalyx of the epithelial cells restricts the exposure between them and the antigen; on the other hand, the large surface area of microvillus allows the epithelial cells to play a key role in antigen uptake. Enzymes or pH changes in the lumen of the gastrointestinal tract modify the chemical properties of the antigens, allowing them to destroy the epitopes before they are processed by the APCs and change the cellular stimulus. |
| Miron & Cristea5 2012 | Review | The best-known function of enterocytes is the chemical processing of food, but they can also induce immunological oral tolerance since they cooperate with cells of the intestinal mucosaassociated lymphoid tissue to maintain a nonreactivity state toward dietary and microbial antigens. The microenvironment of the intestinal lamina propria triggers the events that polarize APCs and activated T-lymphocytes; for this reason, the contribution of enterocytes modifies this microenvironment and maintains the balance between suppression and stimulation of the inflammatory response. |
| Chan et al.6 2004 | Review | Immune tolerance is divided into central and peripheral tolerance. In central tolerance, the ectopic antigens expressed in the thymus medulla produce Treg cells and eliminate self-reactive cells. However, in the thymus, there is no 100% negative selection of self-reactive thymocytes, so peripheral tolerance becomes a secondary mechanism of tolerance induction. In the periphery, tolerance is mainly induced by the interaction between dendritic cells and regulatory T cells. |
| Jiang & Chess7 2006 | Review | 3% of the T-cell precursors entering the thymus survive positive and negative selection (cells with high avidity for self-peptides). CD4+ regulatory T cells express a large amount of FOXP3 transcription factor, whose mutation in humans generates autoimmune diseases and inflammatory disorders such as immune dysregulation, polyendocrinopathy, enteropathy, and X-linked syndrome (IPEX). However, the overexpression of this factor has an immune response suppressing activity, which is evidence of its involvement in immune regulation. |
| Sakaguchi et al.8 2008 | Review | The regulatory T cell marker CD25 is a component of the high-affinity IL-2 receptor, which promotes high levels of FOXP3 and activated T cell apoptosis. In humans, CD25 deficiency is associated with severe autoimmunity and allergies, and its manifestations are indistinguishable from IPEX syndrome, which is generated by the FOXP3 mutation. |
| Jaramillo et al.9 2006 | Review | In infections, regulatory T cells (Treg) limit the immune response against pathogens and are activated directly by the pathogen or products of the infection. In cases of HIV infection, in the periphery, decreased levels of FOXP3 mRNA and regulatory T cells are observed, as well as an inverse relationship between the amount of Treg cells and the appearance of the immune reconstitution inflammatory syndrome. On the other hand, in the case of infections caused by human T- lymphotropic virus-1 (HTLV-1), the tax gene may exert an inhibitory effect on the expression of FOXP3, which generates alteration in the function of Treg cells. |
| Dubois et al.10 2009. | Case-control | Oral tolerance initiates in gut-associated lymphoid tissues by dendritic cell-mediated deletion of Ag-specific T cells and is completed systemically by CD4+CD25+ T cells. This suggests that orally administered biotherapies that increase the susceptibility of effector T cells to the suppressive response of Treg may be of great value for the treatment of autoimmune and inflammatory diseases. |
| Scalea et al.11 2016 | Review | The inhibitory mechanism of Treg cells is believed to be mediated mainly by 4 actions: release of soluble inhibitory factors, cytolysis, metabolic dysregulation, and altered dendritic cell function. |
| Coombes et al.12 2007 | Review | A population of CD103+ mesenteric lymph node dendritic cells induces the development of FOXP3+ Treg cells. |
| Ashton-Chess et al.13 2007 | Review | Tolerance biomarkers are necessary to measure the susceptibility of patients to respond to tolerance-inducing regimes, to diagnose tolerance after induction or weaning patients from immunosuppressive drugs, and to predict when they will no longer be effective. Techniques such as ELISA, flow cytometry and polymerase chain reaction and DNA microarrays are used to detect biomarkers that predict the risk of graft rejection. |
| Koelman et al.14 2000 | Case-control | Oral exposure to HLA molecules in seminal fluid in pregnant women who have practiced oral sex reduces the risk of preeclampsia, as there is a correlation between swallowing sperm during oral sex and a lower incidence of preeclampsia. Since pregnancy and transplantation have several similarities, it is proposed that the induction of allogeneic tolerance to the fetus' paternal HLA molecules may be critical for reducing the risk of pre-eclampsia. Recent studies suggest that exposure, particularly oral exposure, to soluble HLA (sHLA) or HLA-derived peptides may induce tolerance to transplantation. Similarly, sHLA antigens present in seminal plasma may induce tolerance to the father's antigens in the mother. |
| Yin et al.15 2018 | Case-control | Oral administration of Tsumura Japan (TJ-35) increases survival time in heart transplantation in murine models and may induce the production of CD4+CD25+Foxp3+regulatory T cells in these models. |
| Yokoyama et al.16 2005 | Case-control | Administration of a cyclooxygenase 2 inhibitor (NS-398) in murine models induced indefinite survival of most fully mismatched cardiac grafts and generated CD4+T regulatory cells. |
| Jin et al.17 2014 | Case-control | Of 34 kinds of Japanese medicinal herbs studied in murine models, 12 prolonged allograft survival. The administration of Sairei-to (TJ-114) and Tokishakuyaku-san (TJ-23) allowed achieving allograft survival indefinitely (MST>100 days). Patients that received Seisinrensiin (TJ-111), Tokishigyakukagoshuyushokyoto (TJ-38), Rikkunshito (TJ-43), Maobushisaishinto (TJ-127), Ninjin-yoei-to (TJ-108), Ryokan-kyomi-shinge-nin-to (TJ-119), Inchingorei-san (TJ-117), Hochuekkito (TJ-41), Kihi-to (TJ-65) and Sinbu-to (TJ-30) also obtained prolonged survival times (MSTs of 28, 22, 16, 14, 14, 13, 12, 9.5, 9 and 9 days, respectively). |
| Ilan et al.18 2010 | Case-control | Oral OKT3 antibody enhances T-cell proliferation, suppresses Th1 and Th17 lymphocyte response, and increases TGF-β and IL-10 factors expression. Accordingly, oral OKT3 antibody offers a new mechanism for the treatment of autoimmune diseases. |
| Ilan et al.19 2000 | Case-control | Chronic graft-versus-host disease (cGVHD) is a serious complication after bone marrow transplant. Murine models that received bone marrow transplants and were previously sensitized with donor splenocytes by mouth showed improved signs of cGVHD. |
| Taur et al.20 2012 | Case-control | After stem cell transplantation, intestinal microbiota changes and there is an increased risk of developing bacteremia. After allogeneic hematopoietic stem cell transplantation, the diversity and stability of the intestinal flora is disturbed, resulting in the proliferation of bacteria associated with the subsequent development of bacteremia. The evaluation of fecal microbiota allows identifying the patients at greater risk of bloodstream infection after transplantation. |
| Tawara et al.21 2013 | Case-control | After analyzing the effect of donor microbiota on the severity of GVHD induced by T cells from germ-free and pathogen-free donors in murine models, it was found that donor microbiota does not alter the expansion and differentiation of alloreactive T cells nor the severity of the disease. |
| Stonc et al.22 1990 | Case-control | Administration of 16,16-dimethyl prostaglandin E2 (DMPGE2), a stable prostaglandin E2 analogue, significantly prolonged survival time of heterotopic cardiac grafts from ACI to LBN rats. It is concluded that DMPGE2 suppresses solid-organ graft rejection, inhibits allogeneic mixed lymphocyte response, and induces donor-specific in vitro hyporesponsiveness. |
Treg: regulatory T-cells; HLA: human leukocyte antigen; IL: interleukin; APC: antigen-presenting cells; MST: median survival time; TGF-β: Transforming Growth Factor Beta.
Source: Own elaboration.
Discussion
The main motivation for carrying out this research was the need to identify the available literature on the treatment provided to patients receiving solid organ transplants, the associated side effects and the new therapies to solve these problems. The results of this review show that, even though there are no human clinical trials on new post-transplant therapies, there are several laboratory animal studies that describe different types of intervention related to this issue.
To better understand these new works, it is necessary to revisit the physiological foundations of immunological tolerance and its relationship with solid organ transplantation, considering that oral tolerance induction is discussed in this work as an alternative therapy to the one currently used.
Oral tolerance
The adaptive immune system is found in vertebrate organisms and is composed mainly of antibody-secreting cells (B cells) and specialized T cells (which have distinct functions in immune response) and can eliminate pathogens and generate tolerance to antigens. Antigens are useful for avoiding attacks against the body's own cells and preventing excessive responses against external antigens.23
Oral tolerance is defined as the suppression of the immune response to antigens that have been previously administered orally.1 This is a form of peripheral tolerance in which attempts are made to treat external agents that come into contact with the body through the mouth as if they were internal components, thus making them part of the individual. In other words, it is a method to induce immune tolerance systemically.24 The term oral tolerance was first used in 1911 when Wells fed chicken egg proteins to guinea pigs and saw that they were resistant to anaphylaxis.25 Although many researchers have tried to reproduce these results,26 they have not succeeded because tolerance is an active immune event that involves multiple factors such as the dosage of the antigen, the human microbiota, and the co-stimulation of these components.3
Mechanisms of action of oral tolerance
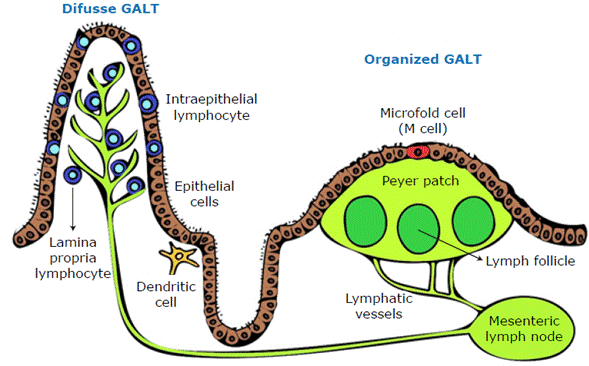
When given orally, an antigen is initially found in gut-associated lymphoid tissue (GALT), which is the largest immune organ in the human body.27 The function of this system is the ingestion and recognition of dietary antigens to avoid unwanted immune responses and protect the body against pathogens, thus allowing for a tolerogenic environment.28 GALT comprises epithelial cells, intraepithelial lymphocytes and lamina propria lymphocytes in the form of lymphoid nodes (known as Peyer's patches) located in in the lowest portion of the small intestine and in the mesenteric lymph nodes (Figure 2).
Another important part of GALT is intraepithelial lymphocytes, which regulate intestinal homeostasis, maintain barrier function, respond to infection, and modify the adaptive and innate immune response. The most important intraepithelial lymphocytes are CD8+T cells. 3
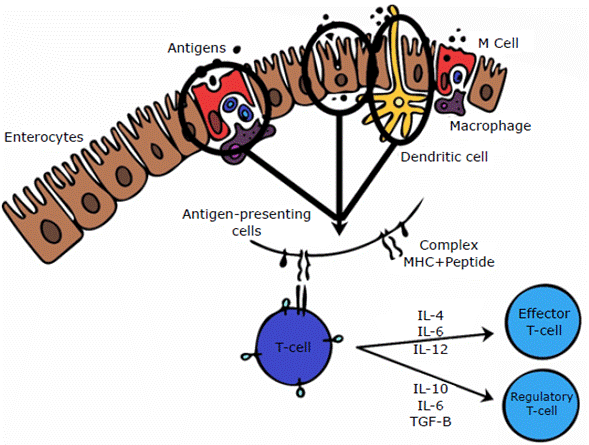
To induce a mucosal immune response, the antigen must gain access to antigen-presenting cells (APCs) by penetrating the mucus layer and the epithelial cell barrier. These antigens are transported through mechanisms, most commonly M cells associated with Peyer's patches,29 which internalize antigenic proteins through phagocytosis and endocytosis, taking them to the extracellular space where they are processed and presented by lymphocytes and macrophages.30 Other mechanisms used by antigens to access APCs is dendritic cell extension into the intestinal lumen31 and through enterocytes (located in Peyer's patches), which capture soluble antigens, processing them and presenting them to the effector cells. 4
The induction of the immune response occurs after processing the antigen captured in the intestinal lumen. To this end, APCs express the antigen through molecules of the major histocompatibility complex, allowing T lymphocytes to recognize it. In addition, depending on the microenvironment, T-lymphocytes can be classified as regulators or effectors; the former are responsible for oral tolerance, while the latter are involved in cytolytic activity32 (Figure 3).
Role of enterocytes
Enterocytes play a key role in immune tolerance because, besides being a mechanical barrier against foreign substances, they react intelligently to the heavy antigenic load of the gastrointestinal mucosa. This type of cell has specialized receptors that recognize pathogens such as Toll-like receptors (TLRs) and NOD-like receptors (NLRs), which are sensors of molecular patterns of bacteria and stimulate the inflammatory mechanisms that activate the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB). 33
With respect to the maintenance of immune tolerance, enterocytes express a limited variety of TLRs (subtype TLR-2) in their apical region,5 which activate the 3-phosphokinase pathway when stimulated with peptidoglycans, which in turn stimulates a negative regulation of NF-kB.34,35
Enterocytes have two main proinflammatory cascades, one mediated by NF-kB, as mentioned above, and the other by p38, a mitogen-activated protein kinase. The inhibition exerted by both pro-inflammatory cascades on the enterocytes is the mechanism that maintains immune tolerance in the intestine. This way, NF-Kb activation produces the downregulation of p38 because the former induces the activation of a protein phosphatase kinase-1 (MKP-1) that dephosphorylates the latter36 and promotes tolerance by inhibiting one of the major pro-inflammatory cascades.
Basic mechanisms of immune tolerance
Immune tolerance is defined as the absence of a specific response against specific actively acquired antigens. Tolerance mechanisms occur via T cells and B cells and can be established centrally (central tolerance) during cell genesis and differentiation, and peripherally (peripheral tolerance) on already differentiated adult cells:6,7
T-cells
Central tolerance. During the embryonic stage and neonatal period, immature cells migrate from the bone marrow to the thymus to express receptors that recognize peptides of the major histocompatibility complex. T cells that recognize these complexes with high avidity survive; this process is known as positive selection.37 In contrast, T cells that weakly recognize such complexes die, and this is known as negative selection,38 which is the main mechanism for regulating self-tolerance;39 the surviving T-cells migrate to secondary lymphoid organs.6 An additional mechanism of immune tolerance is the modification of the T cell receptor to allow it to bind to interleukine-7 (IL-7)-like cytokines, which induces the formation of regulatory T cells (Treg) or lymphocytes.8Peripheral tolerance. Mechanisms of peripheral tolerance include: anergy, immunological ignorance, clonal deletion, active suppression and Treg cells.
Anergy: It occurs when, despite the existence of the first signal (CD3) to trigger T-lymphocyte response - in other words, the recognition of the MHC+ peptide junction-, there is no second signal (co-stimulation), which causes no response from the T-lymphocytes.8
Immunological ignorance: It occurs in the absence of T-cell activation due to low concentrations of the antigen, which does not induce the first signal.40
Clonal deletion: It refers to lymphocyte apoptosis by caspase activation.41
Activa suppression: Cell activity is suppressed through the secretion of inhibitory cytokines such as transforming growth factor B (TGF-β) and interleukin 10 (IL-10) by Treg cells. 8
Treg cells: They are dominantly responsible for controlling the immune response. 9
B cells
Central tolerance. B cells are formed, expressed, and matured in the bone marrow; however, they can be highly avid (leading to clonal deletion) or moderately avid (leading to receptor editing) due to autoantigens. 42
Peripheral tolerance. Surviving B cells migrate to the periphery and those that are highly avid for autoantigens are eliminated through the intrinsic apoptosis pathway. Low-affinity B cells enter into partial anergy because if they are exposed to high doses of the antigen, they can be re-recruited. 43 It should be noted that the dose of antigen taken orally is a fundamental determinant of the immune response: low doses induce tolerance via Treg lymphocytes, while high doses induce anergy or clonal deletion.25,44,45
One of the best ways to understand oral tolerance is the identification of the role of dendritic cells, retinoic acid and CD103+ lymphocyte differentiation cluster in their induction. 3 The expansion of dendritic cells in vivo improves oral tolerance, since they induce the expression of Treg FOXP3+ cells when found in the mucosa by means of TGF-β and retinoic acid signaling pathways.3 Dendritic cells in the mucosa can be divided into two types: CD103+ (tolerogenic) and CD103- (non-tolerogenic). Tolerogenic cells produce retinoic acid and induce FOXP3+ Treg cells if given TGF-β. 12,46
There are other innate immune cells that are relevant for oral tolerance. On the one hand, macrophages are found in the lamina propia of the intestine and produce IL-10. On the other hand, dendritic cells are found in the gut; they produce p-catenin, which stimulates the production of retinoic acid, IL-10 and TGF-Β; trigger the proliferation of Treg cells; and inhibit the response of effector T cells.3
The importance of the tolerance mechanisms in the intestinal mucosa described above lies in understanding that regulatory lymphocytes do not travel from other lymphatic organs to the site of response, but that oral antigens induce the formation of Treg cells.
When dendritic cells recognize antigens in the gastrointestinal tract, the inflammatory response istriggered, resulting in up to 80% anergy and deletion for each antigen. This is known as primary response and its effector organ is the liver; the secondary immune response occurs in the mesenteric nodules with the presentation of antigens by the tolerogenic dendritic cells.10
Role of Treg cells in oral tolerance
Treg cells are the most widely studied cells in relation to oral tolerance induction.11,47 Currently, operational tolerance after transplantation, which is understood as the long-term survival of a graft in the absence of maintenance immunosuppressive therapy, 13,48 is mediated by an antigen-specific response caused by FOXP3 expressing CD4+ CD25(high) regulatory T cells. These cells control the immune response against the donor's alloantigens. 49
Of all Treg cells, the most relevant for transplantation are CD4+ CD25+ regulatory T cells22 and FOXP3+ transcription factor expressing cells.50 The introduction of this transcription factor into CD4+ CD25+ T cells gives them the ability to suppress and amplify the transcription of specific regulation genes. 49
Type 3 and 4 immunoglobulin transcriptase enzymes favor the induction of co-stimulatory molecules in CD4+ CD25+ Treg cells. This makes the latter tolerant to donor antigens by stimulating CD4+ T cells and natural killer cells, which produce positive feedback on CD4+ CD25+ Treg cells to express the FOXP3 protein. As a result of this expression, an antigenic microchimerism is formed, and it allows immune tolerance and prevents rejection of the transplanted organ (Figure 4).51
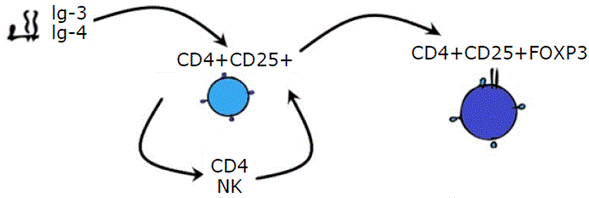
Ig: immunoglobulin; CD: cluster of differentiation; NK: natural killer cell; FOXP3: forkhead box P3. Source: Own elaboration.
Figure 4 Antigenic microchimerism.
The main advantage of immune tolerance is its specificity since it maintains the immune response against neoplasms and microorganisms. However, there is a decreased response to alloantigens in the transplanted organ.49
It is known today that the administration of oral antigens induces the production of Treg cells, particularly, induced CD4+ CD25+ FOXP3+ Treg cells, natural CD25+ FOXP3+Treg cells, Tr1 and CD8+ cells.3 Regarding these subclasses, Tr1 cells act through cell contact, unlike the others, which do so through suppressive cytokines such as IL-10 and TGF-β. Tr1 cells suppress the response of virgin T cells, the expression of co-stimulatory cells, and the secretion of pro-inflammatory cytokines by APCs (Figure 5). (49
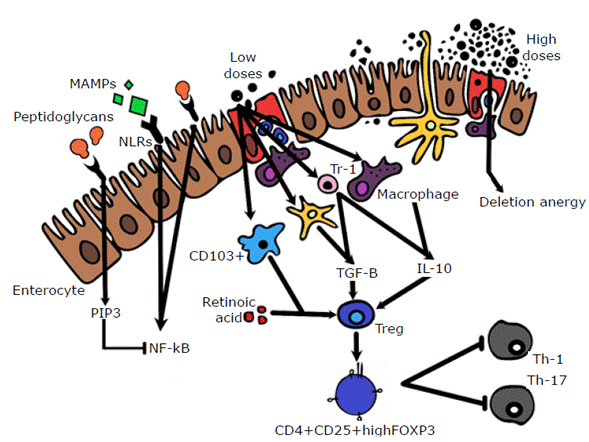
CD: cluster of differentiation; TGF-β: transforming growth factor-beta; IL: interleukin; Treg: regulatory T lymphocyte; Th: helper T lymphocyte; MAMPs: microorganism associated molecular pattern; TLR: Toll-like receptor; FOXP3: fork-head box P3; PIP3: phosphatidylinositol 3-phosphate; NF-Kb: nuclear factor kappa-light-chain-enhancer of activated B cells; NLRs: NOD-like receptors. Source: Own elaboration.
Figure 5 Mechanisms of immunological tolerance.
Because Treg cells are known to mediate tolerance induction and maintenance of their effect over time, researchers such as Scalea et al.11 state that adoptive transfer of this type of receptor-derived cells may lead to stable graft tolerance.
During solid organ transplantation, the immune system generates a response that is attributed to rejection, which is mediated by antibodies, T cells or vascular disturbances; in this response, antibody rejection is the worst prognosis.52,53
Immunosuppressive drugs used in transplants produce a large number of side effects,54 including nephrotoxicity, malignancy, hypertension, diabetes and infections. Cytomegalovirus infection is one of the most common. 55-57
Studies and usefulness in transplants
In Colombia, the first kidney transplant was performed at the Hospital San Juan de Dios in Bogotá in 1963.58 Since then, transplantation surgeries and the amount of places where these procedures are performed have increased, and today there are about 25 hospitals authorized to perform solid organ transplantations in the country.59 Nevertheless, several obstacles must be faced to achieve successful transplants, for example, graft rejection due to lack of immunological tolerance.
As described above, one of the methods for achieving immunological tolerance is the oral route since the intake of alloantigens or soluble human leukocyte antigen from the donor allows the immune system to make an initial recognition through the APCs and the major histocompatibility complex in the gastrointestinal tract. This way, when the transplant is done, the recipient's immune system will not trigger a rejection response, as tolerance to the donor's antigens will already have been generated.
One of the most promising results in induction of immune tolerance to a transplanted graft was found in a study by Kelman et al.,14 which describes what happens to the immune system during pregnancy. These authors state that there is tolerance between the molecules of the human leukocyte antigen system of the fetus and the immune system of the mother and report a correlation between oral sex and swallowing sperm and a lower incidence of preeclampsia. Said results allow us to presume that the oral route is a useful mechanism to induce tolerance.
Most of the evidence found comes from murine models with heart, kidney, cornea, and bone marrow transplants, as shown in Table 2.
Table 2 Studies of oral tolerance induction and their results.
| Sample | Trasplanted organ | Intervention | Results | Findings | Ref. |
| Murine | Cornea | Corneal epithelial cells (2x106 cells/dose) | Not reported | Increase in IL-10 and decrease in IL-2 and IFN-y | 60 |
| Corneal and splenic epithelial cells (2x106 cells/mL) | 20 days | Not reported | |||
| Murine | Heart | Control * | 13 days | Not reported | 61 |
| Sairei-to (TJ114) (2 g/kg/day) | >30 days | ||||
| Murine | Heart | Control * | 7 days | Not reported | 15 |
| Tsumura Japan (TJ-35) (2 g/kg/day) + Licorice | 18 days | ||||
| Tsumura Japan (TJ-35) (2 g/kg/day) | 20.5 days | Increase in CD4-CD25 FoxP3 | |||
| Splenic cells sensitized with Tsumura Japan (TJ-35) | 63 days | ||||
| Murine | Bone marrow | Splenic cells + Lactococcus lactis | Not reported | Increase in FoxP3 | 62 |
| Murine | Bone marrow | Ciprofloxacin (50 mg/kg) | 77 days | Decrease in inflammation and markers in the liver and intestine in 40% of the patients who received Ciprofloxacin and in 70% of those who received Lactobacillus rhamnosus | 63 |
| Lactobacillus rhamnosus | 77 days | ||||
| Murine | Heart | Control * | 8 days | Increase in IL-4 | 16 |
| COX-2 inhibitor (20 mg/kg) | 11 days | Increase in IL-10 | |||
| Sensitized spleen tissue (20 mg/kg) | >100 days | Increase in IL-4 e IL-10 | |||
| Sensitized spleen tissue (2 mg/kg) | 18 days | Increase in Treg cells | |||
| Sensitized spleen tissue (0.2 mg/kg) | 0 days | Not reported | |||
| Murine | Heart | Tokishakuyaku-san (TJ-23) (2 g/kg) | >100 days | Not reported | 64 |
| Tokishakuyaku-san (TJ-23) (0.2 g/kg) | 27 days | ||||
| Tokishakuyaku-san (TJ-23) (0.02 g/kg) | 8 days | ||||
| Murine | Heart | Inchingorei-san (TJ-117) (0.5 g/kg/day) | 16 days | Increase in CD4, CD25 and Foxp3 | 17 |
| Inchingorei-san (TJ-117) (1 g/kg/day) | >100 days | ||||
| Inchingorei-san (TJ-117) (2 g/kg/day) | 12 days | ||||
| Humans | NA | Anti-CD3 (0.2 mg) | Not reported | Increase in TGF-β | 18 |
| Anti-CD3 (1 mg) | Increase in IL-10 | ||||
| Anti-CD3 (5 mg) | |||||
| Anti-CD3 (0.2 mg) + b-glucosylceramide (7.5 mg) | 43% TH1 lymphocyte suppression | ||||
| Anti CD3 (1 mg) + b-glucosylceramide (7.5mg) | 41% TH17 lymphocyte suppression | ||||
| Murine | Heart | Sairei-to (TJ114) (2 g/kg/day) | >100days | Leukocyte infiltrates and mild obliterative vasculopathy | 65 |
| Sairei-to (TJ114) (0.2 g/kg) | 41 days | ||||
| Sairei-to (TJ114) (0.02 g/kg) | 7 days | Not reported | |||
| Sairei-to (TJ114) (0.002 g/kg) | 7 days | ||||
| Distilled water | 7 days | ||||
| Splenic cells sensitized with Sairei-to (TJ114) (2 g/kg) | >100 days | Leukocyte infiltrates and mild obliterative vasculopathy | |||
| Control * | 7 days | Not reported | |||
| Murine | Spleen | Splenic cells (50 mcg) | Not reported | Increase in IL- 10 | 19 |
| Splenic cells (1.9x106 cells/dose/day) | 18 days | Decrease in IFN | |||
| Murine | Cornea | Corneal epithelium | 20 days | Decrease in IL-4 and increase in IL-10 | 66 |
| Corneal epithelium + endothelial cells | 18 days | Increase in TGF-β | |||
| Murine | Kidney | Splenic cells (1x108 cells/ 300 mcL) | 46 days | Increase in CD4 and CD8 | 67 |
| Murine | Kidney | Splenic cells infused into the portal vein | 33.6 days | Not reported | 68 |
| Control * | 8 days | ||||
| Splenic cells single dose (1x107 cells) | 13 days | ||||
| Splenic cells multiple dose (1x107 cells) | 20 days | ||||
| Anti-CD4 monoclonal antibody (200 mcg/ dose) + Splenic cells | 18 days | ||||
| Murine | Heart | Anti-CD4 monoclonal antibody (15 days before) + Splenic cells | 26 days | Not reported | 69,70 |
| Anti-CD8 monoclonal antibody (200 mcg/dose) | 100 days | ||||
| CD8 Antibody (200 mcg/dose) + Splenic cells | 52 days | ||||
| Anti-CD4 monoclonal antibody (200 mcg/ dose) + Splenic cells (1x107 cells/ dose) | 62 days | ||||
| Murine | Skin | Anti-CD4 monoclonal antibody (200 mcg/ dose) | 18 days | Not reported | 71 |
| Splenic cells | 19 days | ||||
| Control * | 12 days |
IL: interleukin; COX: cyclooxygenase; Treg: regulatory T lymphocyte; CD: cluster of differentiation; FOXP3: forkhead box P3; NA: not applicable; IFN: interferon; TGF-β: transforming growth factor-beta; TH: helper T lymphocyte.
* No intervention.
Source: Own elaboration.
Probiotics, herbal medicine, COX-2 inhibitors, monoclonal antibodies and CD4 lymphocytes from the donor are some of the antigens that have been used to induce immunological tolerance.
The use of probiotics in transplant recipients was considered an alternative when changes in the microbiota were evident in the subjects (humans and mice) that rejected the graft. In this regard, Finney et al.72 found increased Bacteroides and Ruminococcus colonies in specimens that rejected liver transplantation in murine models; in contrast, Taur et al.20 observed decreased Bacteroides colonies in human models that presented acute rejection to kidney transplantation. Therefore, the use of probiotics has been proposed as a way to modify the microbiota and the immune system, not only at a local level but also at a systemic level. 2,21
Probiotics have demonstrated good survival rates after bone-marrow transplantation in murine models because its administration stimulates both anti-inflammatory and pro-inflammatory signals. 62 These microorganisms are responsible for maintaining the integrity of the epithelial barrier of the gastrointestinal tract by stimulating immunoglobulin A and the secretion of mucus and defensins, and by altering the adhesion of bacteria to the epithelium.
In 2004, Gerbitz et al.63 found that the administration of Lactobacillus rhamnosus before transplantation improved long-term survival and reduced rejection associated with decreased CD3 levels.
The COX enzyme is responsible for prostaglandin secretion, which has two isoforms: COX-1, a constitutive enzyme, and COX-2, induced by the action of cytokines and tumor promoters. In 1990, Stonc et al.22 proved that the use of COX-2 inhibitors prolongs the survival time of cardiac grafts in mice, and in 2005, Yokayama et al.16 noted that its use prolongs transplant survival beyond 100 days versus 11 days in the control group.
Given their immune system modulating characteristics, medicinal plants are another type of antigen used throughout history to sensitize the donor to transplants. 64 For example, in Asia, these plants have been used as alternative therapy for different diseases in humans for more than 3 000 years; in the case of transplants, it has been reported that their use in murines with heart transplants increases the survival time of the graft. 17
Medicinal plants have been studied at the molecular level, finding that they are made up of multiple components. For example, the administration of Tokishakuyaku-san, also known as Tsumura TJ-23, at a minimum dose of 2 g/kg in murine models has yielded good results in terms of cardiac graft survival. 64
Oral tolerance induction studies in humans also include the use of anti-CD3 monoclonal antibodies (OKT3). Its immunological effects in peripheral blood were the suppression of Th1 and Th17 lymphocyte response, increased expression of Treg cell markers, increased TGF-β/IL-10 and decreased expression of IL-23/IL-6 in dendritic cells without side effects. 18 Likewise, studies in mice have shown how the intake of CD4 lymphocytes from the donor decreases the induction of pro-inflammatory cascades and increases IL-10 levels in cases of spleen transplantation. 19,65-67
Finally, combined therapy of CD4 lymphocytes and anti-CD3 orally demonstrated promising results in kidney and heart transplantation in murine models by prolonging the survival of the grafts by more than 100 days. 68,69,71 None of the interventions described above have been studied in humans, so despite having a physiological basis, these results cannot be extrapolated or presumed to be safe in a human context. However, the use of oral immunological tolerance induction strategies opens a door to the study of new practices to treat chronic diseases and manage side effects of immunosuppressive drugs, immune transplant rejection, autoimmune diseases, among others, which may generate high-quality evidence to create novel strategies in the field.
Conclusions
There are many immunological mechanisms that underlie transplantation tolerance. The oral route is an alternative for inducing tolerance in transplant patients, since it eliminates the adverse effects that current immunosuppressive therapy entails.
Although it is possible to demonstrate the viability of oral tolerance for immunological induction and its possible usefulness in the transplant field, it should be noted that, according to the literature, there are no human clinical trials to ensure that oral immunological tolerance induction strategies are safe. Therefore, there is no high-quality evidence to infer that these strategies are safe in humans.