Dear Editor:
An intra-individual somatic variation (ISV) is described as the genetic difference among different tissues of the same individual. ISV increases with age, may not manifest a defined phenotype, and is associated with neurological, hematological, and immune disorders, especially with cancer.1
To analyze possible ISV in Mexican patients with colorectal cancer (CRC), we studied the polymorphism rs669 (c.2998 A>G, p.Ile1000Val) of the A2M gene, which encodes for the alpha-2 macroglobulin protein, a protease inhibitor involved in tumor progression and proliferation.2 This variant is located near a thioester site, which is necessary for the inhibitory function of the protein.
For the present study, the variant was selected based on previous research conducted by Ramirez-Plascencia.3 She determined that the frequency of the G allele in peripheral blood was 0.35 in 146 healthy individuals from western Mexico, who had an average age of 42 years within a range of 19 to 48 years; 62% of the participants were men. The variant was found to be in equilibrium according to the Hardy-Weinberg assumption that analyzes the distribution pattern of genotypes (p=0.098).
Prior informed consent and immediately after surgical resection, tumor tissue, healthy tissue adjacent to the tumor and peripheral blood were obtained from 62 patients with CRC that had not received chemotherapy or radiotherapy treatment (Table 1). The average age in this group was 63 years with a range of 34 to 96 years.
Table 1 Clinical-pathological characteristics of 62 patients with colorectal cancer.

Source: Own elaboration.
After histopathological diagnosis, DNA was extracted from the tumor tissue and the healthy tissue adjacent to the tumor using the High Pure PCR Template Preparation kit, and from peripheral blood using the DTAB-CTAB method (dodecyltrimethylammonium bromide - cetyltrimethylammonium bromide).4 The variant was identified by PCR-RFLP (polymerase chain reaction - restriction fragment length polymorphism) with the primers Forward 5'-GGAGACATATTAGGCTGC-3 ' and Reverse 5'-CTGAAACCTGGGAAATCC-3', and with the enzyme Mbol. Enzyme digestion products were analyzed in 6% polyacrylamide gel stained with silver nitrate.
The AA, AG and GG genotypic frequencies in peripheral blood were 0.40, 0.52 and 0.8, respectively, and 0.40, 0.53 and 0.7, respectively, in tumor tissue. Statistical comparison with Fisher's exact test between the groups showed no statistically significant difference (p=0.96), but 3 (5%) patients with different genotype between tumor and blood were identified. This result was the same after repeating the test twice.
The genotype in the healthy colorectal tissue adjacent to the tumor was identified to determine if the variation corresponded to the somatic alterations associated with the tumor tissue. This led to establish that the peripheral blood and healthy tissue genotypes were identical, as opposed to what was found in the tumor tissue; this also confirmed the ISV exclusive of the tumor in the 3 patients with different genotypes (Figure 1).
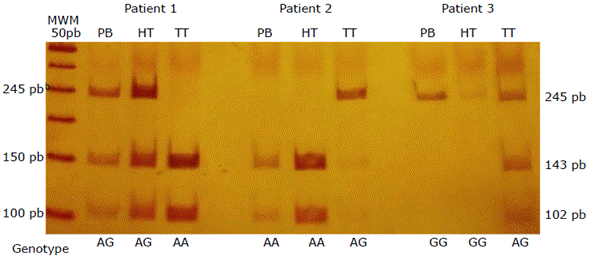
Polyacrylamide gel at 6% stained with silver nitrate. PB: peripheral blood; HT: healthy tissue; TT: tumor tissue; MWM: molecular weight marker. Source: Own elaboration.
Figure 1 Intra-individual somatic variation in 3 patients with colorectal cancer evidenced by a different genotype in tumor tissue.
After analyzing the clinical data of ISV patients, it was found that all were male, and 2 were under 40 years of age and their cancer had not been diagnosed as hereditary. However, finding ISV in only 3 of the 62 participants was, due to their small number, a limitation to establish a relationship with age, sex, tumor location and degree of progression.
The difference of the tumor genotype in patient 1 (Figure 1) can be explained by hemizygosity, which means that the genotype interpreted as homozygous AA in tumor tissue may be only one allele A instead, i.e. loss of the allele G in the tumor since the peripheral blood and healthy tissue show the AG genotype. At this point, it should be noted that hemizygosity frequently originates from chromosome segment deletion or total chromosome loss, alterations that are part of the chromosomal instability pathway, considered the most frequent molecular pathway in CRC development.5
Regarding alterations of chromosome 12, according to the Mitelman database,6 monosomy 12 is one of the main findings in adenocarcinomas, which supports the feasibility of hemizygosity for rs669 polymorphism in patients with CRC. Nevertheless, the genotype in the tumor tissue of the 3 patients with ISV could have originated from the incorrect incorporation of nucleotides during DNA replication or from the effect of endogenous or exogenous mutagens with inadequate DNA repair, facts that have been described as the most frequent routes of de novo mutations.7 Continuous exposure to toxic agents and their high cell proliferation is also influential in the case of colorectal tissue, as demonstrated in the study in human autopsies by O'Huallachain et al.,8 where ISV was determined based on the great variety of findings in tissues of constant division such as the intestine.
This research shows how the study of ISV enriches the knowledge of genetic diversity among tissues by changing the concept of identical genome in somatic cells for a dynamic model, which can impact on genetic diseases such as cancer.9 It should be noted that ISV have been described in patients with neoplasms, mainly in relation to variants associated with drug responses. Thus, the analysis of variants of the MTHFR gene in patients with colon cancer published by Rai et al.10 shows that the A1298C polymorphism only had identical genotypes in 45.81% of patients when the tumor and normal tissue adjacent to the tumor were compared. However, other authors describe ISV in cancer in terms of genotype discrepancy between peripheral blood and tumorous colorectal tissue: Marsh et al.11 report frequencies of 1.1% in 1 139 comparisons made in 44 patients in which 28 polymorphisms were analyzed, Van Huis-Tanja et al.12 report frequencies of 1.4% in 1 418 genotypes of 11 genotyped single nucleotide polymorphisms in 149 patients, and Balboa et al.13 claim that the frequencies are up to 22% among the genotypes of 10 variants studied in 65 patients.
The 5% ISV finding for the rs669 polymorphism of the A2M gene in CRC patients did not show significant differences between the tissues analyzed, but contributed to show the genetic variation associated with cancer, even in passenger genes or "low-penetrance" genes such as A2M. Future research on other variants with a larger sample are expected to provide more representative evidence of ISV in CRC.














