Introduction
In Colombia, the first suspected Zika virus (Zikv) infection report was made on September 22, 2015 in the municipality of Turbaco, with a subsequent exponential increase, and a total of 341 confirmed cases registered in epidemiological week 43 of 2015. The virus was found in 36 regions, led by the department of Bolívar with 81 cases.1 In addition, the epidemiological bulletin of week 24 of 2016, issued by the Colombian National Institute of Health (INS), showed how this pandemic became a public health problem after reporting 8 500 confirmed cases and 86 446 suspected cases based on clinical manifestations; 70% of these suspected cases were reported in 502 municipalities where the infection was later confirmed in a laboratory.2
The clinical signs and symptoms of Zikv infection consist of conjunctivitis, headache, fever (less than 38.0°C), mild myalgia and arthralgia, rash or maculo-papular exanthema with or without pruritus, anorexia, emesis, diarrhea, abdominal pain, lower limb edema, and retro-orbital pain (with less frequency), which last from 2 to 7 days. Fever usually lasts a couple of days and is accompanied by a rash. In general, infection is asymptomatic and self-limited in up to 80% of subjects.3,4 However, complicated and rare forms may occur, such as those affecting pregnant or lactating women, or those leading to neurological and ophthalmological complications.5
Adequate treatment of Zikv requires dealing with both the syndrome and its complications, which is why it is important to understand the cycle of the virus.6,7 Currently, the therapeutic approach is only symptomatic, where drugs, including analgesics and antipyretics, are used. Recently, therapies using antiviral drugs that have been proved to be efficient in the treatment of dengue fever have been attempted, mainly due to the similarity between this condition and Zikv; nevertheless, their efficacy has not yet been proven.8,9
According to the INS, any person with a clinical suspicion of Zikv infection should receive clinical and support management, including adequate hydration and rest. Non-steroidal anti-inflammatory drugs (NSAIDs) and acetylsalicylic acid (ASA) must not be used due to the risk of bleeding, which is similar to that of dengue. Thus, acetaminophen is the only recommended and reliable medication for treatment in the acute phase of the disease.10
Currently, in Colombia, there is limited information on the epidemiological behavior, clinical characteristics and treatment of Zikv infections, as well as on the variables associated with the impact of this disease on the population diagnosed with it.10,11 Therefore, this study was conducted to describe clinical manifestations, sociodemographic characteristics, treatment, and adverse clinical outcomes in patients diagnosed with Zikv disease at a primary care hospital in a municipality of Colombia, from January 1 to July 25, 2016.
Materials and methods
A descriptive cross-sectional study was conducted. The sample consisted of patients who attended medical consultation at a primary care hospital in the municipality of La Virginia, Risaralda, Colombia, and who were initially diagnosed with Zikv based on physical examination and the symptoms reported. The diagnosis was later confirmed through a RT-PCR test. All 254 patients were diagnosed with Zikv disease from January 1 until July 25, 2016, when the INS reported the Zika epidemic was over.12
All cases reported in the primary care hospital during the study period were analyzed. The units of analysis were the medical records of the patients who met the inclusion criteria.
Inclusion criteria
Patients of any age and sex who attended medical consultations at a primary care hospital in a municipality of Colombia, who were initially diagnosed with Zikv based on clinical evidence (cases registered in epidemiological report cards of the National Public Health Surveillance System, SIVIGILA), and whose diagnosis was later confirmed through a RT-PCR test from January 1 to July 25, 2016.
Variables
A database was constructed from the information found in the medical records of the patients who met the inclusion criteria. The records were retrieved from the hospital database and the epidemiology department information recording systems by using ICD10 codes and the SIVIGILA epidemiological report cards for reporting Zikv cases. After the hospitals consented the use of the data, they were entered into an information collection instrument designed through the Epi Info 7.1 software (for Windows). The following variables were obtained:
1. Sociodemographic variables:
Sex, age (years), marital status (single/others), place of origin (urban/rural) and type of enrollment to the General System of Social Security in Health (SGSSS) [subsidized, contributory, linked regime (please note that the latter is for the population that does not have the means to pay for health insurance coverage and is not yet enrolled in the subsidized regime)].
2. Clinical variables:
General: pregnant (yes/no), type of medical service consulted (emergency or outpatient consultation). Clinical manifestations (yes/no): rash, pruritus, arthralgia, headache, conjunctivitis, asthenia, myalgias, retro-ocular pain, diarrhea, abdominal pain, edema, emesis, bleeding, lymphadenopathy, neurological deficit, rhinorrhea, dehydration.
Vital signs: respiratory rate (breaths per minute), heart rate (beats per minute), temperature (°C), blood pressure (mmHg). The following values were considered as cut-off points for defining abnormalities: tachycardia: heart rate >100 beats per minute; tachypnea: respiratory rate >20 breaths per minute; fever: temperature >38.0°C; hypotension: systolic blood pressure <90 mmHg and diastolic blood pressure <60 mmHg. Laboratory tests (yes/no): blood count, C-reactive protein, urinalysis, and others.
Comorbidities (yes/no): hypothyroidism, hyperthyroidism, obesity, hypertension, diabetes, human immunodeficiency virus infection (HIV), epilepsy, allergies, chronic obstructive pulmonary disease (COPD), chikungunya virus infection, dengue, malaria, smoking, depression.
3. Pharmacological variables:
Did the physician prescribe any medication? (yes/no): Name of the medication; characteristics of the medication such as formulation, concentration and dosage were considered for each medication. Consumption of medications (self-medication) for symptom management prior to consultation (yes/no): Name of the medication.
Comedication (yes/no): name of the medication. In this study, comedication is defined as the use of medications for patient-based pathologies different than Zikv symptoms.
4. Adverse clinical outcomes (yes/no): anemia (hemoglobin <11.5 mg/dL), hematocrit (%), leukopenia (<4 000 leukocytes/mL), leukocytosis (>10 000 leukocytes/ mL), neutrophilia (>8 000 neutrophils/mL), neutropenia (<2 000/mL), thrombocytopenia (< 150 000/mm3), gestational or fetal/neonatal adverse outcomes (yes/ no, and name of the adverse outcome), and hospitalization (yes/no).
Statistical analysis
The Epi Info 7.0 statistical software for Windows was used to analyze the data. Descriptive statistics were performed: frequencies and proportions were used for categorical variables, and measures of central tendency and dispersion were used for continuous variables. X2 tests were carried out to compare categorical variables. In addition, p values, OR, and confidence intervals were included. A binary logistic regression model was applied where the adverse clinical outcomes associated with Zikv infection were considered as a dependent variable; adverse clinical outcomes that were associated in a statistically significant way in the bivariate analyzes were regarded as independent variables. A p<0.05 value was determined as a level of statistical significance.
Reduction of selection biases was achieved by analyzing the information of the all population included in the study; in contrast, a p<0.05 value was established for information and confusion bias, which is stricter than the Hosmer-Lemeshow test to reduce overestimation of results.
This study is considered as a "risk-free research" according to Resolution No. 8430 of 1993, issued by the Colombian Ministry of Health,13 which establishes scientific, technical, and administrative standards for health research. Likewise, the present study followed the ethical principles for medical research of the Declaration of Helsinki.14 Additionally, it was granted ethical approval by the Bioethics Committee of Universidad Tecnológica de Pereira as stated in Minutes 23 of December 12, 2016.
Results
In total, 254 patients were diagnosed with Zikv infection from January 1 to July 25, 2016 were found. Diagnosis was more common in women (male-to-female ratio = 2.2:1), and the mean age of the sample was 29.4±18.1 years. The main sociodemographic characteristics of the population are shown in Table 1.
Table 1 Socio-demographic characteristics of patients with Zika virus treated at a primary care hospital in Colombia, 2016.
| Variable | n | % | ||
| Sex | Female | 174 | 68.5 | |
| Male | 80 | 31.5 | ||
| Age | <5 years | 18 | 7.1 | |
| >60 years | 19 | 7.5 | ||
| Socio- | Marital | Single | 188 | 74.0 |
| demographics | status | Other | 66 | 26.0 |
| Origin | Urban | 214 | 84.3 | |
| Rural | 40 | 15.7 | ||
| SGSSS* | Subsidized | 238 | 93.7 | |
| Others | 16 | 6.3 | ||
* General System of Social Security in Health.
Source: Own elaboration.
The most frequent clinical manifestations included rash, pruritus and arthralgias. The main symptoms, clinical characteristics, comorbidities, and laboratory results are presented in Table 2.
Table 2 Clinical characteristics, comorbidities and laboratory test results of patients with Zika virus infection treated at a primary care hospital in Colombia, 2016.
| Variable | n | % | ||
| Rash | 206 | 81.1 | ||
| Pruritus | 142 | 55.9 | ||
| Arthralgias | 100 | 39.4 | ||
| Headache | 97 | 38.2 | ||
| Conjunctivitis | 96 | 37.8 | ||
| Asthenia | 85 | 33.5 | ||
| Myalgias | 55 | 21.7 | ||
| Retroocular Pain | 39 | 15.4 | ||
| Clinical manifestations | Diarrhea | 33 | 13.8 | |
| Tachypnea | 29 | 11.4 | ||
| Abdominal pain | 19 | 7.5 | ||
| Tachycardia | 17 | 6.7 | ||
| Edema | 17 | 6.7 | ||
| Fever | 10 | 3.9 | ||
| Emesis | 10 | 3.9 | ||
| Hypotension | 9 | 3.5 | ||
| Bleeds | 7 | 2.8 | ||
| Lymphadenopathy | 4 | 1.6 | ||
| Neurological deficit | 2 | 0.8 | ||
| Pregnant woman | 52 | 20.5 | ||
| Clinical | Service | Emergency room | 99 | 39.0 |
| characteristics | Outpatient | 155 | 61.0 | |
| Referral to tertiary care hospital | 8 | 3.2 | ||
| Hypertension | 22 | 8.7 | ||
| Allergic | 22 | 8.7 | ||
| Obesity | 21 | 8.3 | ||
| Diabetes | 16 | 6.3 | ||
| Comorbidities | Asthma | 16 | 6.3 | |
| Smoker | 12 | 4.7 | ||
| Gastritis | 10 | 3.9 | ||
| Cancer | 7 | 2.8 | ||
| Hypothyroidism | 6 | 2.4 | ||
| Others | 17 | 6.8 | ||
| Hemogram | 190 | 74.8 | ||
| Laboratory tests | C Reactive Protein | 53 | 20.8 | |
| Urinalysis | 36 | 14.2 | ||
Source: Own elaboration.
In addition, an average of 4833.33 leukocytes per mm3 (95%CI: 4 699.00-5 000.00) was reported in the medical records of the patients included in this research. Regarding hemoglobin, hematocrit, and platelets count, an average of 12.33 g/dL (95%CI: 12.00-13.00), 37.33% (95%CI: 36.00-40.00), and 286 312.9 per mm3 (IC95%: 198 000.0-303 000.00) was found, respectively.
Pharmacotherapy
Five different pharmacological groups were prescribed, namely, antipyretics, antihistamines, rehydration salts, corticosteroids and NSAIDs, being acetaminophen the most commonly used (90.1%), followed by corticosteroids and NSAIDs (3.5% and 0.7%, respectively). Drugs prescribed by attending physicians for the treatment of Zikv infection are depicted in Table 3.
Table 3 Prescribed drugs for the treatment of patients with Zika virus infection in a primary care hospital in Colombia. 2016.
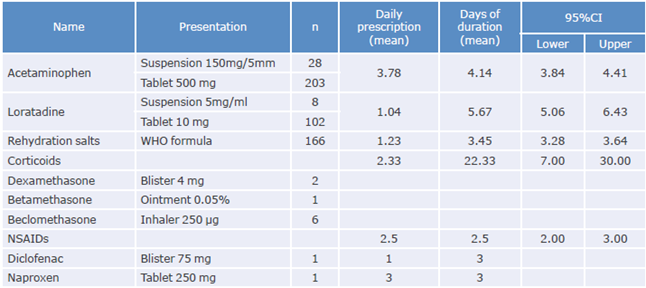
CI: confidence interval; WHO: World Health Organization; NSAIDs: Nonsteroidal anti-inflammatory drugs.
Source: Own elaboration.
Regarding the use of drugs for treating symptoms prior to consultation at the hospital (self-medication), the medical records of the patients described that 84 (31.9%) reported the use of antihistamines; 21 (8.3%), opioids; 17 (6.7%), antibiotics; 16 (6.3%), antiemetics; 14 (5.5%), vitamins; and 13, anti-ulcers (5.1%). Likewise, 59 more patients (16.3%) used other self-medicated drugs for symptoms management (16.3%) prior to consultation.
146 patients (57.4%) used 1 comedicated drug, 14 (5.5%) used 2 comedicated drugs, and only 2 (0.8%) used 3 or more comedicated drugs. Use of comedication was not described in 92 patients (36.2%).
Adverse clinical outcomes
Regarding variables associated with adverse clinical outcomes, alterations in blood analysis were the most frequent, being anemia the most common finding (18.5%). The main adverse clinical findings are described in Table 4.
Table 4 Adverse clinical outcomes in patients with Zika virus infection treated at a primary care hospital in Colombia, 2016.
| Variable | n | % |
| Anemia | 47 | 18.5 |
| Leukopenia | 17 | 6.7 |
| Neutrophilia | 11 | 4.7 |
| Leukocytosis | 9 | 3.6 |
| Neutropenia | 8 | 3.1 |
| Other | 5 | 2.0 |
| Guillain-Barré | 2 | 0.8 |
| Premature rupture of membranes | 2 | 0.8 |
| Abortion | 1 | 0.4 |
| Pneumonia | 1 | 0.4 |
| Thrombocytopenia | 0 | 0.0 |
| No complication | 151 | 59.0 |
| Total | 254 | 100 |
Source: Own elaboration.
Multivariate analysis
A binary logistic regression in the associated variables of the bivariate analysis (described in Table 5) allowed finding that factors like being a woman (p=0.045, OR: 2 410.0, 95%CI: 1.018-5.703), being pregnant (p=0.000, OR: 11.077, 95%CI: 4.329-27.938), and being hospitalized (P=0.006, OR: 4.995, 95%CI: 1.578 -15.280) were associated with a greater probability of having adverse clinical outcomes.
Table 5 Unadjusted analysis of the variables associated with adverse clinical outcomes in patients with Zika virus infection treated at a primary care hospital in Colombia, 2016.
| Clinical | No clinical | OR | 95%CI | p | |||
| Variable | alterations % (n) | changes % (n) | Lower | Higher | |||
| Sex | Female | 39 (68) | 61 (106) | 5.061 | 2.373 | 10.793 | 0.000 |
| Male (r) | 11 (9) | 89 (71) | |||||
| Emergency (r) | 52(51) | 48 (49) | |||||
| Treating service | Outpatient consultation | 17 (26) | 83 (129) | 5.272 | 2.961 | 9.387 | 0.000 |
| Pregnant women | Yes (r) | 81 (42) | 19 (10) | 20.040 | 9.187 | 43.716 | 0.000 |
| No | 17 (35) | 83(167) | |||||
| Gastritis | Yes (r) | 80 (8) | 20 (2) | 10.145 | 2.101 | 48.975 | 0.000 |
| No | 28 (69) | 72 (175) | |||||
| Conjunctivitis | Yes (r) | 18(17) | 82 (79) | 0.351 | 0.190 | 0.650 | 0.001 |
| No | 38 (60) | 62 (98) | |||||
| Retro-ocular pain | Yes (r) | 15 (6) | 85 (33) | 0.369 | 0.148 | 0.921 | 0.027 |
| No | 33 (71) | 67 (144) | |||||
| Hospitalization | Yes (r) | 69 (18) | 31 (8) | 6.445 | 2.663 | 15.600 | 0.000 |
| No | 26 (59) | 74 (169) | |||||
CI: confidence interval; r: reference.
Source: Own elaboration
Discussion
This study identified the main clinical manifestations and sociodemographic characteristics of patients diagnosed with Zikv infection disease in the municipality of La Virginia, Risaralda, in 2016, as well as the characteristics of the treatment provided to them in a primary care hospital. Similar studies carried out in Colombia and Brazil4,15,16 have reported a higher frequency of Zikv infection disease in women, but these findings should be contrasted with the percentages of asymptomatic Zikv.
In this regard, in 2016, the Zika virus response epidemiology and laboratory teams of the CDC published a report on the adequate monitoring of people with suspected Zika virus disease and the interpretation of zika virus antibody test results17, stating that interpretation of said results may be difficult in women, particularly in pregnant women, due to cross-reactivity with other flaviviruses, which may hinder the proper identification of the infecting virus.17 Currently, and despite this finding, there is not enough information to explain why Zikv disease is more frequently found in women and why there is a higher clinical manifestation in this population.
In the present study, the average age in both men and women was 30 years, which is similar to what other studies have reported. For example, Vargas et al.,18 in a series of cases of women who gave birth to neonates diagnosed with microcephaly in Pernambuco, Brazil, reported an average age of 25 years (range: 16 to 41 years). Likewise, in a descriptive study carried out in Rio de Janeiro and that is similar to the research presented here, an average age of 37 years (range: 9 to 60 years) was described,16 which suggests that Zikv infection is more frequent in people who are in their twenties or thirties. Likewise, based on the age distribution data obtained for the inhabitants of the municipality of La Virginia, it is possible to conclude that people in their twenties or thirties constitute the largest age group in this area, which may explain why the proportion of Zikv infection cases in this age range is higher.19
Regarding symptoms, Paz-Bailey et al.,15 in a study on the persistence of Zikv in body fluids, reported that rash (93.8%), pruritus (80.7%) and fever (78.8%) were the most common symptoms. Likewise, Brasil et al.16 described that rash (97%), pruritus (79%), prostration (73%), headache (66%) and arthralgia (63%), with or without associated edema, were the symptoms most frequently manifested. These data agree with what was found in the present study, since rash, pruritus and arthralgias were the most common symptoms according to the medical records analyzed.
Considering this information, a physician should always suspect Zikv infection in all patients that experience a rash, pruritus, and arthralgias and are living or visited Zika endemic areas. However, if a patient experiences these three symptoms, it does not necessarily mean that he is infected with Zika, since they are also associated with Chikungunya disease; so, in order to avoid confusions, intense joint pain or absence of conjunctivitis can help differentiate the diagnosis.10
Fever was not observed in most of the patients included in this study (3.9%), a finding that has been previously informed in other studies; for example, one study carried out in Brazil reported that fever, with a maximum duration of one day, was only present in 36% of the study population.16 However, a high frequency of fever events in the pregnant population has been reported,20 and this may be a topic that future studies should address to determine whether fever in Zikv infected pregnant women is associated with their hormonal characteristics, or if it leads to the development of complications.
In this sense, in 2016, Vargas et al.,18 in a study conducted in pregnant women diagnosed with Zikv, reported that 55.8% of them experienced fever, which highly differed from the occurrence of fever cases in the general population included in the same study (3.9%). Yet, there was no statistically significant association of this symptom with the development of complications.18 In this study, even pregnant women who were asymptomatic during the viremic episode developed complications, highlighting the importance of monitoring pregnant women and children exposed to the Zika virus.18
Regarding laboratory results, Brasil et al.16 reported a mean white blood cell count of 4 590 cells/mL (range: 2 240 to 11 570 cells/mL), a mean platelet count of 201 000 x mm3 (102 000 to 463 000 x mm3), and mean hematocrit levels of 41.2% (33.2% to 50.3%), while Paz-Bailey et al.15 reported an average of 5 200 leukocytes/mL (range 2 100 to 40 000 cells /mL), a mean platelet count of 216 000 cells/mm3 (80 000 to 373 000/ mm3), and mean hematocrit levels of 42.2% (30.9% to 51.9%).15 These values are similar to those found in our study, although it should be noted that these results were not associated with the adverse clinical outcomes observed in the patients included in the present study.
Nevertheless, Boyer-Chammard et al.21 published a case report series where severe thrombocytopenia was associated with Zikv infection in 7 patients, while Dirlikov et al.22 reported that less than 1% of the patients in their study died after developing severe thrombocytopenia. Consequently, it is not clear what role hemogram alterations play in patients infected with Zikv, thus evidencing the lack of studies addressing the association between blood count and clinical alterations during the course of the disease or afterwards (follow-up consultations).
On the other hand, the present study found 2 patients with Guillain-Barré syndrome but, since this figure is not significant, and considering that these patients were referred to a tertiary care hospital once the diagnosis was made, other probable causes of this pathology are unknown. Therefore, it was not possible to establish a causal association between having Guillain-Barré syndrome (GBS) and being infected with Zikv. In this regard, Mata et al.23 described that in 57 patients with GBS and other neurological alterations who reported having an infectious process up to 31 days before the onset of neurological symptoms, 30 (52.63%) were classified as probable cases of Zikv infection, a causal association that has been informed often in several studies around the world, including Colombia.24-26
In the present study, 11 patients received NSAIDs and corticosteroids for treating symptoms, yet no association between using these drugs and clinical alterations was observed. In this sense, in a systematic review, Passi et al.27 reported that treatment of Zikv infected patients aims to manage and provide care for symptoms that include pain, fever and pruritus, by prescribing rest and large amounts of fluid intake to prevent dehydration. Furthermore, these researchers state that medications such as aspirin, NSAIDs, corticosteroids, or other alike should not be prescribed until dengue or thrombocytopenia diagnoses are ruled out to avoid bleeding or other complications.
Taking antihistamines, opioids, and antibiotics for managing symptoms before the consultation (self-medication) was considered as comedication in this study. However, only opioids could be indicated for pain management and antihistamines for rash and pruritus management, as antibiotics are ineffective against Zikv infection, thus its indication is useless in these cases.27 To the best knowledge of the authors of the present study, this is the first report analyzing comedication and treatment in patients diagnosed with Zikv infection that has not found any association with any clinical outcome.
Some limitations of this study include its design, since it is not possible to determine what reasons led health care professionals to prescribe medications that are not suitable for the management of Zikv infection symptoms, or the motives behind comedication, by only analyzing medical records. Another limitation is the source and characteristics of the information obtained, since these data were extracted from medical records of adult individuals of any age who attended medical consultation at a primary care hospital in a Colombian municipality and most of them were enrolled in the subsidized regime of the Colombian health care system, so the results found here can only be extrapolated to populations with similar characteristics. Information bias, typical of cross-sectional studies, was reduced using variables with unique options and with previous training for the selection of the variables of the clinical records.
Conclusion
The clinical manifestations described here are similar to those reported in other populations. However, fever was not a frequent symptom (apart from pregnant women); further studies on this symptom in different population subgroups, such as the elderly, children, and pregnant women, are required. Furthermore, inappropriate pharmacological management practices that can lead to complications in this population, such as bleeding, were observed in some cases. Thus, educational interventions on the proper prescription of medications for treating this disease aimed at general physicians working in Zika affected areas must be implemented to improve the prognosis of these patients.















