Introduction
Celiac disease (CD) has a prevalence of 1% in the general population and between 3% and 12% in patients with type 1 diabetes mellitus (DM1).1 This difference has been associated with some genetic and environmental particularities of these patients, such as increased genetic predisposition linked to HLA-DQ2 or HLA-DQ8 markers, activation of some KIR (killer immunoglobulin-like receptor) genes, and specific features of the intestinal microbiota.2
CD is characterized by severe small intestinal mucosa atrophy, which leads to impaired digestion and malabsorption of nutrients and, consequently, produces gastrointestinal disorders and alterations in anthropometric parameters.3 CD is often under-diagnosed due to the scarce knowledge among health professionals of this condition and because of the variability of its clinical presentation, which includes signs and symptoms such as anemia, constipation, diarrhea, abdominal pain and distention, stunt growth, among others.
Detecting tissue antitransglutaminase IgA antibodies (IgA-TGT), which is mainly done quantitatively, may suggest CD. In other words, measuring IgA-TGT can be used as a screening method to determine the risk of having this disease, as well as to confirm its diagnosis with the support of other serological and duodenal biopsy tests.
The objectives of the present study were to identify the positivity for the detection of IgA-TGT in children with DM1 treated in two pediatric endocrinology centers from Bogotá, Colombia, and to describe the gastrointestinal (GI) symptoms, anthropometric nutritional status and gluten intake of the participants.
Materials and methods
A descriptive cross-sectional study was performed on a convenience sample of children aged 0-18 years with a diagnosis of DM1 and no other diagnosed autoimmune diseases. All patients who attended two outpatient pediatric endocrine centers between January 2011 and June 2016 and between July 2016 and January 2017 were selected.
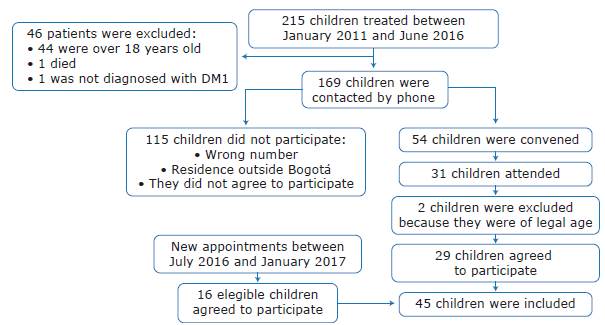
For the inclusion of participants, the parents of children diagnosed with DM 1 were contacted by telephone to invite them to participate in the study. Figure 1 describes the communication and sample collection process.
The parents of the children who agreed to take part in the study signed an informed consent, and participants older than 8 years signed an assent. The research followed the ethical principles for medical research on human subjects of the Declaration of Helsinki4 and took into account the local regulations of the Colombian Ministry of Health established in Resolution 8430 of 1993.5 Furthermore, the research was approved by the ethics committees of the Universidad El Bosque through Minutes No. 005-2016 of March 8, 2016, and Universidad Nacional de Colombia through Minutes No. 021-27715 of December 10, 2015.
A survey on GI symptoms suggestive of CD was carried to collect the data following the European Society of Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) guidelines. This instrument assesses the presence of symptoms such as vomiting, nausea, abdominal pain and distension, flatulence, constipation, diarrhea, and irregular bowel habits, among others. It also investigates aspects such as bowel habits, the characteristics of bowel movements according to the Bristol scale6 and the frequency of bowel movements. The survey was sent by e-mail.
Based on the protocol of the International Society for the Advancement of Kinanthropometry, weight, height, skin folds (triceps, biceps, subscapular and suprailiac) and cephalic, waist and brachial circumferences were measured. The analysis and interpretation of weight-for-height, height-for-age and BMI-for-age indicators was carried out using the Anthro and Anthro Plus programs of the World Health Organization (WHO).7,8 Fat percentage was determined with the Siri formula, using the Nutritional Application tool of the Spanish Society for Pediatric Gastroenterology, Hepatology, and Nutrition, and was interpreted according to the percentiles for age.9 Waist circumference was determined following the same process10
The participants received an e-mail with the appointment confirmation, as well as the food anamnesis form so that the child and their guardian could record food intake during the three days prior to the appointment. The information had to include portion size, which was corroborated with the eNasco food modules on the day of the consultation. The dietary analysis was performed using the DIAL program based on the average of intake during those three days.11 To calculate gluten intake (g/ day), the daily grams of protein ingested from wheat, rye, oats and barley were taken as reference and multiplied by the conversion factor 0.8.12
Anti-IgA antibodies were measured by lateral flow immunochromatography using the BiocardTM Celiac® test, a laboratory test that allows screening for CD and in which IgA is not measured to determine positivity for tissue transglutaminase.13
The information was tabulated in a database in Microsoft Excel 2016 and then exported to SPSS version 22.0 for the corresponding descriptive statistical analysis. Categorical variables, absolute values and percentages were used. Means and standard deviations were reported for continuous quantitative variables (σ), while medians and interquartile ranges were used for interval variables. The Shapiro-Wilk test was applied to assess whether data were compatible with a normal distribution. In case data were not normal, non-parametric tests, such as Pearson's chi-square, were used to compare proportions and the Wilcoxon-Mann-Whitney test was used to compare means and medians; when the distribution was normal, tests such as Fisher's t-Stu-dent were used to compare proportions and ANOVA to compare means and medians. A 95% confidence level (p<0.05) was considered for all statistical tests.
Results
Forty-five children diagnosed with DM1 and an average age of 10.7±4.1 years were included and assessed between January 20l1 and June 2016 and between July 2016 and January 2017. The average age at which children were diagnosed with DM1 was 6.6 years and the average time from diagnosis to assessment was 3.5 years. 53% of them were boys and the distribution by age group was as follows: preschoolers under 5 years: 13.3%; school children between 6 and 10 years: 31.1%; and adolescents between 11 and 18 years: 55.6%.
Although the test was not conclusive in two patients, none of the participants showed qualitative positivity for IgA-TGT. Only 7 children (15.6%) did not report GI symptoms; in those who did, the most frequent symptom was flatulence (48.9%), followed by abdominal pain (28.9%), nausea (26.7%), abdominal distension (20.0%), constipation (11.1%), diarrhea (8.9%), and vomiting (4.4%). The consistency of the stools in 68.9% of the participants was between types 3 and 4 according to the Bristol scale, with an average frequency of 1.8±1.3 times per day.
Regarding height-for-age, 55.6% of patients were between +1 and -1 σ and 25% between -1 and -2 σ; 3 children had low height-for-age. About body mass index (BMI), 60% of the children were between +1 and -1 σ and 31.1% were overweight (>+2 σ). 48.8% of the participants were in the normal body fat percentile ranges, although 46.3% presented some degree of excess fat. With respect to waist circumference, 59.1% were within the 25th-75th percentiles and 15.9% were above the 75th percentile.
Of the 45 participating children, only 38 completed the food intake forms; in them, the average gluten intake was 5.29±3.02g/day, which is equivalent to the daily consumption of a ±69g portion of crackers or a ±97g portion of white bread. Although there was no significant difference between groups, there was a tendency towards increasing gluten intake as age increased.
Discussion
None of the participants tested positive for IgA-TGT according to the lateral flow immunochromatography test, for which a sensitivity of 96.7% and a specificity of 93.5% have been reported.14 A previous study conducted in Valle del Cauca (Colombia)15 found that 10.4% of 115 children with DM1 tested positive for IgA-TGT, while a study conducted in Cuba reported that only 1.2% of 595 children with the same condition tested positive for this antibody.16
The ELISA test determines positivity for IgA-TGT when the values of these antibodies are >5o U/mL; sensitivity of 92% and specificity of 100% have been reported for this tool.17 There are several studies that have reported positivity using this method: Landaeta et al.18 described positivity of 3.4% in 118 Venezuelan children, Brandt et al.19 reported positivity of 21% in 19 children from the state of Pernambuco (Brazil), Araújo et al.20 reported 10.5% positivity in 354 children in the city of Recife (Brazil), Al-Hussaini et al.21 reported 24.5% positivity in 106 Saudi children, Joshi & Madvariya22 found 15.49% positivity in 71 children in Western India, Bhadada et al.23 reported 11.1% positivity in 189 children from northern India, Al-Sinani et al.24 found 17% positivity in 103 Omani children, and Honar et al.1 reported 14% positivity in Iranian children.
In the present work, this test did not yield any results in two patients, which could be explained by an IgA deficiency or because the IgA antibody titer was low in the sample collected. In these cases, it was considered that their anthropometric nutritional status was adequate and that the only reported GI symptom was flatulence, which are characteristics similar to those of the majority of the population analyzed.
On the other hand, Baker et al.25 found that 42% of evaluated diabetic patients were diagnosed with CD 10 years after the onset of DM1, while Bhadada et al.23 found that the diagnosis was made 5 years later. In the present study, the children were diagnosed with DM1 on average 3.5 years before the time of assessment, so even though the result for IgA-TGT was negative for most participants, they should be tested annually (as a screening procedure) to determine if they are positive in the future.
Regarding GI symptoms, the study by Costa-Gomes et al.,26 published in 2016 and conducted on a sample of 111 Brazilian children diagnosed with DM1, found abdominal pain (30.6%) and abdominal distension (28.8%) as the most common symptoms, which is consistent with the study by Bhadada et al.23 conducted in India. In the present study, the results coincide with what these authors found and what is reported in most research works: abdominal pain is one of the most frequent GI symptoms.
Concerning gluten intake, Hoppe et al.27, in a study conducted in Danish children, reported the following average intake values: 1.79 g/day, 3.74 g/day, 5.22 g/ day, 6.74 g/day and 7.4 g/day for children 6-7 months of age, 8-9 months, 10-11 months, 12-24 months and 25-36 months, respectively. The average gluten intake established in the present study was around 5.29 g/day, therefore, in some patients, it is lower than that reported by Hoppe et al.,27 which may be due to the fact that eating patterns in Denmark are different and the intake of gluten source foods is usually higher due to the unavailability of other cereals. In Colombia, according to the 2010 ENSIN,28 92.5% of the population consumes rice or pasta daily and 76.1% eats bread, arepas (round patty made of corn flour) or cookies, so it can be said that other cereals such as rice or corn are used; this, in turn, indicates that the intake of sources of gluten in the country is lower.
Joshi & Madvariya,22 in a study conducted in India with children aged 0-18 years and diagnosed with DM1, found stunting in 18.2% of the sample. In Colombia, according to the ENSIN 2010,28 13.2% of children under 5 years and 10% of children between 5 and 17 years have low height. However, in the present study, this variable was lower than the national average, as only 3 children had low height; the gluten intake of these participants was below the average consumption and their BMI was normal. Still, since the height of the children's parents was not known, it was impossible to determine whether the delay was due to a family characteristic.
No child had a deficit in the BMI indicator. On the contrary, 26.7% were between > + 1 and ≤+2 σ and 4.4% between >+2 and ≤+3 σ, which is above the figures reported in the ENSIN 2010,28 where 4.8% of children under 5 and 17.5% of those over 5 were above +2 σ. This is also in line with the data published by da Costa et al.29 who found in a 2016 study that 30.3% of 195 Brazilian children diagnosed with DM1 had BMI between >+1 and ≤+2 and 9.7% between >+2 and ≤+3. Similarly, Luczyhski et al.,30 in a sample of 500 Polish children with diabetes, found that 30.2% were overweight or obese, similar to what has been reported in the present study.
As for waist circumference, which has been associated with excess weight, of the 25 children over 10 years of age, 14 were above the 75th percentile; 71.4% were females. Other studies29,31 have associated puberty with increased risk of obesity in children with DM1 due to decreased insulin sensitivity, peripheral glucose metabolism and exogenous hyperinsulinism, which, together with the anabolic effect of insulin, generates lipogenesis. In addition, and in agreement with the findings of this research, Wysocka-Mincewicz et al.32 reported that the female sex is a risk factor for obesity in adolescents with DM1.
To measure the levels of gluten intake, a factor of 0.8 was used and all foods considered to be sources of this protein (pasta, bread, crackers, and oat) were included. However, some foods that may contain it, such as sausages, sauces, additives, among others, could not be included in the calculation of the reported total because their exact content was unknown.
While no children was positive for IgA-TGT, the American Diabetes Association and ESPGHAN recommend monitoring children with DM1 regularly, regardless of the presence or absence of symptoms, considering the characteristics of this population and the increased risk of autoimmune diseases such as CD.
In summary, the present study is an approach to understanding, on the one hand, the risk of presenting CD in children with DM1 and, on the other, the anthropometric nutritional status, the presence of GI symptoms and gluten intake in this population. These results seek to sensitize parents and health professionals directly involved (endocrinologists, gastroenterologists, and nutritionists) about the importance of implementing periodic screening to detect early the risk of having CD. In this context, more studies, preferably cohort studies, need to be conducted in Latin America with diabetic children to establish the frequency of CD in the region and thus make early diagnoses that help improve quality of life and avoid associated complications.
Conclusions
According to the results obtained, none of the participants tested positive for IgA-TGT, even though all had a higher risk of developing CD because they had DM1.
Likewise, it was identified that the most frequent GI symptom was flatulence, that most children had an adequate anthropometric nutritional status according to the ranges of normality established by the WHO, and that the average gluten intake was 5.29±3.02 g/day.
Explanatory note
This manuscript derives from the master's thesis entitled "Estudio CED3: Detección cualitativa de anticuerpos IgA contra la transglutaminasa tisular (AtTG), en niños con diabetes tipo 1 en Bogotá" (CED3 study: Qualitative detection of IgA antibodies against tissue transglutaminase (AtTG) in children with type 1 diabetes in Bogotá.)33