Introduction
Worldwide, neglected tropical diseases (NTDs) are caused by protozoan, bacterial and helminth infections, and Chagas disease (CD) is one of the three most important NTDs caused by protozoa and transmitted by vectors.1 In addition, it constitutes a major public health problem in Latin America, especially in endemic continental countries. Also, it has been estimated that approximately 6 million individuals are infected with Trypanosoma cruzi in 19 Ibero-American countries, with an annual incidence of 28 000 cases, in addition to the occurrence of sporadic cases of natural transmission in the United States.2
In 2006, the Southern Cone Intergovernmental Commission to Eliminate Triatoma infestans and Interrupt the Transmission of Transfusional Trypanosomiasis3 (IN-COSUR-Chagas) certified that Brazil had successfully interrupted vector-borne transmission of T. cruzi by T. infestans in all endemic states, the main triatominae in the country and the main responsible for the transmission of this infectious disease.4 However, in Brazil, CD is still the main NTD in terms of morbidity and mortality, since, besides premature death, it also causes physical disability.5,6 It has been estimated that 1.9 to 4.6 million people in the country are infected with T. cruzi, that is, 1.0 to 2.4% of its total population, and that out of these, approximately 540 000 to 1.8 million individuals have cardiovascular or digestive diseases. In addition, it has been reported that in Brazil, CD causes almost 6 000 deaths per year.7,8
There are two phases of CD: the acute phase and the chronic phase, and in both phases it can be symptom free, mild or severe. In the acute or initial phase, the infection can be severe in 3 to 5% of infected people, causing cardiac complications and may lead to sudden death.9 In the chronic phase, 40 to 90% of people do not have symptoms, and their electrocardiograms (ECGs) will be normal or slightly altered, yet their serological and/or parasitological tests will be positive.9,10 During the chronic phase, which may last decades or even the lifetime of the infected individual, most patients have no Chagas-related symptoms (a phase known as chronic indeterminate), but approximately 20% to 30% will develop cardiac complications and digestive complications affecting the esophagus and the colon.8,9
However, the clinical signs and the severity of CD may vary depending on the geographic area and the strain of the parasite.11 Other comorbidities that may affect the severity of the infection and the development of these complications, such as diabetes mellitus and systemic hypertension, should be also considered.
In the Marília region, located in the state of São Paulo, Brazil, T. cruzi infection used to be highly prevalent. This situation was historically associated with coffee farming activities in rural areas and with the expansion of cities in the region as a result of the construction of railroads, which opened up unexplored territories of Western São Paulo.12,13
Thus, considering the high number of CD cases recorded in the region of Marília before the campaigns to eradicate the main vector of T. cruzi in this region were carried out, the present study aims to characterize the clinical and epidemiological profile of patients in the chronic phase of CD treated at a reference center of São Paulo (State), Brazil.
Materials and methods
Cross-sectional descriptive study. The medical records of 62 patients in the chronic phase of CD treated at the Cardiology outpatient clinic of the Hospital das Clínicas de Marília between February and October 2016 were reviewed. This hospital is a referral health institution that provides cardiology care services to patients from all municipalities belonging to the DRS IX-Marília-SP (Regional Health Department IX-Marília-São Paulo) area.
The following variables were analyzed: sex, age, time elapsed after the disease was diagnosed, symptoms and clinical signs of CD, associated comorbidities, electrocardiographic alterations, medications used by the patients, and cardiac alterations based on ECGs results. Functional classification of CD followed the Second Brazilian Consensus on Chagas Disease guidelines8: B1, cardiac involvement without congestive heart failure (CHF), altered ECG, and left ventricular ejection fraction (LVEF) ≥45%; B2: cardiac involvement without CHF, altered ECG, and LVEF <45%; C: altered ECG and compensated heart failure (HF); D: altered ECG and refractory HF.
For age analysis, patients were classified into two groups: adults aged <60 years and those aged between 60 to 84 years.
Continuous numerical variables were described using central tendency (mean) and dispersion (standard deviation) measures. Categorical numerical variables were described using absolute (f) and relative (%) frequencies and were dichotomized based on the following options: presence (1) or absence (0) of clinical manifestations or alterations. A student's' t test and a Fisher's exact test were performed to compare means between men and women, and to determine the association between qualitative variables, respectively. Also, the Spearman's rank correlation coefficient was used to analyze the correlation between categorical variables. A logistic regression analysis was used to verify the effect and odds ratio of the independent variables on the probability of occurrence of the different types of clinical manifestations of CD. The percentage of variation in the probability of the dependent variable explained by the variation of the independent variables in the logistic regression model was analyzed by means of Nagelkerke's R2. All statistical analyses were performed using the IBM SPSS Statistics Software for Windows, version 19.0 (Armonk, NY: IBM Corp.). A significance level of 5% was considered for all analyses.
Before participating in the study, all individuals signed a free and informed consent form. Also, the study was conducted following the ethical principles for medical research involving human subjects established by the Declaration of Helsinki14 and in accordance with the research ethics standards outlined by Resolution 466 of December 12, 2012, issued by the National Health Council of Brazil.15 This research was approved by the Research Ethics Committee of Famena - Faculdade de Medicina de Marília (Faculty of Medicine of Marília) as stated in the CAAE (Certificado de apresentação para Apreciação Ética - Submission certificate for ethical evaluation) No. 11565712.8.0000.5413 (12/19/2012).
Results
Participants' age ranged from 31 to 83 years, 24.2% were younger than 60 years old, and 75.8% older than 60. There were no significant differences between men and women and between age groups (Table 1).
Table 1 Distribution of patients in the chronic phase of Chagas disease treated at the Cardiology outpatient clinic of Hospital das Clínicas de Marília from February to October 2016 (n=62) according to sex and age group.
| Sext | Age group† | f | % * | p-value |
|---|---|---|---|---|
| Female (n=32) | <60 years | 8 | 25.0 | 0.879 |
| ≥60 years | 24 | 75.0 | ||
| Male (n = 30) | <60 years | 7 | 23.3 | |
| ≥60 years | 23 | 76.7 |
* Comparison of mean according to the student's' t test
† Association between sex and age group according to the Fisher's exact test.
Source: Own elaboration.
In addition, the clinical manifestations of CD reported in the medical records that were reviewed are shown in Table 2.
Table 2 Clinical manifestations of Chagas disease in the study population (n=62) according to sex and age group.
| Clinical manifestations | Age group (years) | Sex | p-value* | |||
|---|---|---|---|---|---|---|
| Female | Male | |||||
| f | % | f | % | |||
| Cardiac complications | <60 | 6 | 75.0 | 5 | 71.4 | 1 |
| ≥60 | 19 | 79.2 | 15 | 65.2 | 0.341 | |
| Esophageal complications | <60 | 1 | 12.5 | 0 | 0 | 1 |
| ≥60 | 4 | 17.4 | 3 | 11.1 | 0.689 | |
| Intestinal complications | <60 | 0 | 0 | 0 | 0 | - |
| ≥60 | 6 | 26.1 | 2 | 7.4 | 0.121 | |
| Indeterminate (asymptomatic) | <60 | 2 | 25.0 | 2 | 28.6 | 1 |
| ≥60 | 2 | 8.7 | 7 | 30.4 | 0.072 | |
* A p-value <0.05, obtained after performing the Fisher's exact test, was considered as a significant association.
Source: Own elaboration.
The analysis of the association between age, sex, comorbidities, functional class of CD, and electrocardiographic alterations in the study population is shown in Table 3. The main comorbidity was systemic hypertension (SH), followed by dyslipidemia and diabetes mellitus. Alzheimer's disease was not reported, and only one woman had a rheumatic disease. Electrocardiographic alterations such as anterosuperior left bundle branch block (ALBBB), altered ventricular repolarization, first-degree atrioventricular block, ventricular extra-systole, atrial fibrillation, left bundle branch block, and volume overload of the left atrium are not shown in Table 3 because they were reported in only one case each, making any statistical analysis impossible. It is noteworthy that despite these alterations were statistically assessed, they were not relevant for the final results of the study.
Table 3 Association between age, sex, comorbidities, functional classification of Chagas disease and electrocardiographic alterations in the study population (n=62).
| Comorbidity, functional class of CD, and electrocardiographic alterations | Age group | Sex | p-value* | |||
|---|---|---|---|---|---|---|
| Female | Male | |||||
| f | % | f | % | |||
| SH | <60 | 0 | 0 | 3 | 42.9 | 0.077 |
| ≥60 | 20 | 83.3 | 9 | 39.1 | 0.003* | |
| DM | <60 | 1 | 12.5 | 0 | 0.0 | 1 |
| ≥60 | 7 | 30.4 | 2 | 7.4 | 0.013* | |
| DYS | <60 | 0 | 0.0 | 1 | 14.3 | 0.467 |
| ≥60 | 10 | 41.7 | 8 | 34.8 | 0.766 | |
| Functional class B1 | <60 | 5 | 62.5 | 4 | 57.1 | 1 |
| ≥60 | 15 | 65.2 | 6 | 26.1 | 0.019* | |
| Functional class B2 ' | <60 | 1 | 12.5 | 1 | 14.3 | 1 |
| ≥60 | 1 | 4.3 | 6 | 26.1 | 0.048* | |
| Functional class C | <60 | 0 | 0 | 0 | 0 | NA† |
| ≥60 | 2 | 8.3 | 3 | 13.0 | 0.666 | |
| RBBB | <60 | 1 | 12.5 | 0 | 0.0 | 1 |
| ≥60 | 7 | 30.4 | 3 | 11.1 | 0.286 | |
| Pacemarker | <60 | 2 | 25.0 | 0 | 0.0 | 0.467 |
| ≥60 | 5 | 20.8 | 3 | 13.0 | 0.701 | |
| RBBB+ALBBB | <60 | 2 | 25.0 | 0 | 0.0 | 0.467 |
| ≥60 | 4 | 17.4 | 0 | 0.0 | 0.109 | |
| AVR | <60 | 1 | 12.5 | 1 | 14.3 | 1 |
| ≥60 | 3 | 13.0 | 3 | 13.0 | 1 | |
CD: Chagas disease; SH: systemic hypertension; DM: diabetes mellitus; DYS: dyslipidemia; RBBB: right bundle branch block; ALBBB: anterosuperior left bundle branch block; AVR: altered ventricular repolarization.
* A p-value <0.05, obtained after performing the Fisher's exact test, was considered as a significant association.
† Functional classification C was not observed in patients under 60 years of age.
Source: own elaboration.
The correlation analysis between type of clinical manifestation of CD, functional class of CD, electrocardiographic alterations, and time of diagnosis, described in Table 4, showed that the longer the time elapsed since the diagnosis was made, the higher the probability of having esophageal and intestinal complications. It should be noted that only values with significant correlations were included. The following codes were used to interpret the correlation: presence of manifestations or alterations (1) or absence of manifestations or alterations (0), as mentioned in the methodology.
Table 4 Correlation between type of clinical manifestation of Chagas disease, functional class of Chagas disease, electrocardiographic alterations, and time of diagnosis in the study population (n=62).
| Functional class, electrocardiographic alterations and time of diagnosis | Cardiac manifestation | Esophageal manifestation | Intestinal manifestation | Indeterminate manifestation |
|---|---|---|---|---|
| Functional class B1 | 0.619† | -0.523† | ||
| Functional class B2 | 0.264† | |||
| RBBB+ALBBB | ||||
| RBBB | 0.297† | -0.251† | ||
| RBBB+VE | ||||
| RBBB+VE+AF | 0.333† | |||
| Time elapsed since diagnosis | 0.439† | 0.319† | ||
RBBB: right bundle branch block; ALBBB: left bundle branch block; VE: ventricular extrasystole; AF: atrial fibrillation.
† a p-value ≤0.05 indicates a significant correlation by the Spearman's correlation coefficient.
Source: Own elaboration.
Table 5 shows the binary logistic regression analysis for the independent variables that showed a significant correlation (Table 4) with the clinical manifestations controlling the effect of sex and age group. However, variables with a Nagelkerke's R2 correlation coefficient >0.30 were considered for the regression analysis. The following codes were used to interpret the regression analysis: presence of clinical manifestations (1) or absence of clinical manifestations (0), sex (0=female; 1 = male); age group (0:<60 years; 1: ≥60 years).
Table 5 Logistic regression analysis of the effect of independent variables in increasing the probability of occurrence of any clinical manifestation of Chagas disease.
| Variables | B | Wald p-value* | Exp(B) | IC 95% for Exp(B) | X2 p-value | Nagelkerke’s R2 | ||
|---|---|---|---|---|---|---|---|---|
| Dependent | Independent | Lower | higher | |||||
| Cardiac manifestation | B1 | 21.53 | 0.998 | <0.001 | <0.0001 | >1 | 0.0001† | 0.545 |
| Age group | 0.65 | 0.495 | 1.92 | 0.29 | 12.56 | |||
| Sex | 0.28 | 0.710 | 1.32 | 0.31 | 5.70 | |||
| Constant | -0.84 | 0.380 | 0.43 | |||||
| Esophageal manifestation | TD | 0.19 | 0.005* | 1.21 | 1.06 | 1.39 | 0.002 † | 0.411 |
| Age group | -0.04 | 0.978 | 0.97 | 0.07 | 12.44 | |||
| Sex | -0.24 | 0.801 | 0.78 | 0.12 | 5.23 | |||
| Constant | -6.05 | 0.002* | 0.00 | |||||
| Intestinal manifestation | TD | 0.14 | 0.008* | 1.15 | 1.04 | 1.27 | 0.002 † | 0.336 |
| Sex | -1.20 | 0.201 | 0.30 | 0.05 | 1.90 | |||
| Constant | -4.34 | 0.002* | 0.01 | |||||
| Indeterminate (asymptomatic) | B1 | -21.04 | 0.998 | 0.0001 | 0.001 | 0.00001 | 0.0001 † | 0.487 |
| Age group | -1.48 | 0.138 | 0.23 | 0.03 | 1.61 | |||
| Sex | 0.70 | 0.391 | 2.01 | 0.41 | 9.91 | |||
| Constant | 0.36 | 0.701 | 1.44 | |||||
B1: functional classification B1; TD: time of diagnosis (in years); B (regression coefficient); x2: Chi-square; R2: Nagelkerke's percentage of variation in the probability of the outcome explained by the model; Exp (B): odds ratio.
* a p-value ≤0.05 indicates a significant effect of each independent variable by the Wald test.
† A p-value ≤0.05 means a significant effect of the model on the variation of the probability of the outcome taking place. The effect was measured using the Chi-square (x2) statistic.
Source: Own elaboration.
Regarding the association between the time of diagnosis and the probability of developing clinical manifestations of CD, it was observed that for every year elapsed since the diagnosis was made, the probability of having a digestive complication increases (Figure 1). In this regard, the probability of developing an esophageal complication is 1.21 times higher for every year elapsed since the diagnosis of CD was made (Figure 1A) (p=0.005).
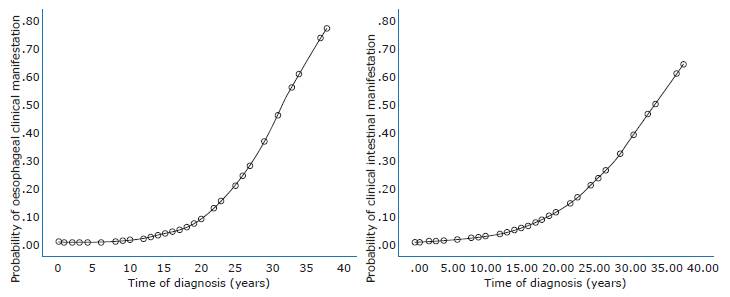
Source: own ellaboration.
Figure 1 Effect of time of diagnosis on the probability of occurrence of the clinical manifestations analyzed. Figure A: esophageal complications; Figure B: intestinal complications.
Regarding the medications used by the study population, 49 patients (79.03%) used at least one medication. Of these, 25 (40.32%) used diuretics; 21 (33.87%), Beta blockers; 16 (25.80%), antiarrhythmic drugs; 15 (24.19%), angiotensin-converting enzyme (ACE) inhibitors; 12 (19.35%), statin; 9 (14.51%), angiotensin II receptor blockers and spironolactone; 7 (11.29%), calcium channel blockers and metformin; 4 (6.45%), glibenclamide, and 3 (4.80%), doxazosin.
Discussion
In Brazil, the success of the T. infestans eradication campaigns has led to a massive decrease in the number of new acute cases of CD; somehow, CD remains a major public health problem.4,8
The impact of the control measures to interrupt the transmission of T. cruzi by the main vector in the country has been described in several studies. For example, Luquetti-Ostermayer et al.16, in a serological survey conducted from 2001 to 2008, reported there were no T. cruzi infection cases in children under the age of five years. In addition, in the state of São Paulo, thanks to the reduction of new acute cases of CD and the reduction of the number of triatomines in its area, the transmission of the disease has been controlled for more than four decades.17,12
Similar to what other authors have described17,18, in the present study, 75.8% of the patients were aged over 60 years. In this regard, Bertanha et al.18 state that increased longevity in patients with CD in Brazil is associated with the current technological advances that have been achieved in terms of CD treatment, given the needs of the new epidemiological profile of Brazilian people as a consequence of the accelerated demographic growth of the elderly in the country.
It should be noted that in our study, four elderly patients were diagnosed with CD within four years before conducting the study. Somehow, despite the fact there were no reports of cardiac or digestive complications suggesting CD, or positive serological tests for CD in their medical records before being referred to the Cardiology service of the Hospital das Clínicas de Marília, it is assumed that these individuals were already in the indeterminate chronic phase of the disease, and their diagnosis was confirmed in our service once they started experiencing CD-related signs and symptoms. However, a recent infection by vector transmission of T. cruzi in these four patients by species of the Triatominae family such as Triatoma sordida19 cannot be ruled out, since this is the most prevalent Triatominae species found in the region where this study was conducted, that is, the state of São Paulo.20
On the other hand, in the group of adults <60 years old, a 31-year-old patient had been diagnosed with CD 3 years before conducting the study. Yet, according to the medical record, the patient was born in the state of Bahía, and then moved to the state of São Paulo and settled in the region of Marília one year after the diagnosis was made. Also, due to the absence of information regarding a possible T. cruzi infection or any blood transfusion the patient may have received, congenital CD or transmission of the infection via blood transfusion were ruled out. However, since the patient was a farmer, it is possible to assume this was a case of vector-borne transmission, provided that in some municipalities of the State of Bahía there are still residual outbreaks of T. infestans.21
In this regard, Brum-Soares et al.11, in a study carried out in the state of Amazonas, reported that 14 patients aged 7 to 36 years tested positive for CD. This reinforces the need to keep working on vector control, to verify blood transfusions processes, and to pay close attention to other routes of transmission, such as oral (food-borne), which currently is one of the most frequent routes of transmission.22
In the present study, there were no statistically significant differences regarding sex between age groups, which is a similar finding to those reported by Borges-Pereira et al.23 and Silva et al.24 in studies carried out in Piauí and Bahía, respectively. In these studies, vector-borne transmission within households was observed in these regions before vector control measures were strengthened. However, in studies conducted in the northern region of Brazil, Brum-Soares et al.11 and Beltrão-Teixeira & Carvalho-de Oliveira25 found that T. cruzi infection was more frequent in men, concluding that CD is associated with the occupation of the infected person, for in this region the disease is mainly transmitted to people who work in the natural habitats of triatomines, and thus are more exposed to the vector transmission route of the disease.
Although in the present study the average time elapsed since the patients were diagnosed with CD was 17 years, this period does not provide accurate information of the exact moment in which the onset of the clinical manifestation of CD occurred, because in many patients' medical records there are reports of tiredness, dysphagia, or constipation that, in some cases, were made before the diagnosis of chronic CD was confirmed by serologic tests.
In our study, cardiac complications were the most frequent clinical manifestation of CD, affecting 72.58% patients, yet no statistical difference between genders was found. These results are similar to those of Almeida et al.26 and dos Santos-Pereira et al.27 who described that cardiac complications were reported in 88.50% of patients in the chronic phase of CD from Campinas (São Paulo) and in 65.30% of those living in the state of Ceará, respectively. However, these results greatly differ from those by Mota et al.28, who found that, in Brazil, 30% of these patients developed any cardiac alteration.
The predominance of cardiac complications observed in our study might be explained by the T. cruzi strains present in the state São Paulo, where Y and Famema strains have been isolated in patients in the chronic phase of CD, as described by Silva & Nussenzweig29 and Martins et al.30 in 1953 and 2003, respectively. Cardiac tissue tropism has been reported for both strains, although experimental studies conducted using these two strains have found differences regarding virulence and pathogenicity between them. Likewise, cardiac tissue tropism has been described in the strains found in other states of Brazil, such as the state of Bahia31, so it is possible to say that the 31-year-old patient included in our study, but who was born in the region of Bahía, may have been infected with any of these strains.
Furthermore, the diversity of T. cruzi strains present in the State of São Paulo, and their ability to cause infection, added to the early diagnosis of complications related to CD and the early onset of treatment might explain why 48.40% patients were classified in functional classes B1 and B2 of CD, and 8.10%, in class C. Also, functional class B1 was more frequent in women, while B2 predominated in men, with statistically significant differences, which is in agreement with the findings of the study by Guariento et al.32, in which men with the disease had a worse prognosis
Based on the ECG results, the cardiac conduction system was affected in patients with altered ECG. Right bundle branch block was the most frequent cardiac alteration, followed by the need to have a pacemaker implanted. In addition, although cardiac complications were more frequent in women, there was no statistically significant difference between sexes, as reported by other studies.24,27,33
Treatments used to minimize the clinical signs and symptoms of CD, such heart rate alterations, probably caused by the destruction of the parasympathetic nervous system34 included the prescription of β-blockers and antiarrhythmic drugs in 33.87% and 25.80% of these patients, respectively. In addition, most of the patients included in the present study were using diuretics (40.32%) and antihypertensives (24.19%), as SH was the most prevalent comorbidity in individuals in the >60 years age group. In this sense, several studies have described an association between CD and hypertension.17,18,35 Out of these patients, 71.80% had cardiac complications, thus confirming that having SH and CD leads to the worsening of the heart function.36
The predominance of SH in females in the >60 years age group presented a significant difference in relation to men, which may be explained by the higher survival rate of women in the general population, and by the hormonal and biological changes that they undergo during menopause.37 Most of the times, these changes result in an increased body mass index,38 which in turn increases the risk of high blood pressure, together with other comorbidities such as dyslipidemia, diabetes, in addition to the infection by T. cruzi. In our study, dyslipidemia was reported in 38.30% of patients in the elderly group (>60 years) and was the second most frequent comorbidity, yet no significant difference was observed between genders, which is similar to the findings of Pereira,39 who reported this condition was observed in 21% of the individuals included in their study.
Furthermore, 12.9% of our patients had diabetes mellitus, which is similar to the prevalence found by Bertanha et al. (10.4%).18 Also, in our study, the majority of patients in the elderly (>60 years) group were women (75.0%), and 30.4% of them had diabetes mellitus. According to some authors, patients in the chronic phase of CD may develop diabetes mellitus due to the excessive production of free radicals caused by T. cruzi infection, which may lead to insulin resistance,40 and due to dysautonomia,41 a condition that can occur in these patients.
Confirming the biological characteristics of the T. cruzi strains existing in the Marília region, the indeterminate form of the disease was the second most prevalent type of CD in the study population, followed by the intestinal and esophageal manifestations types. However, the linear relationship analysis showed that the time of diagnosis can significantly influence the probability of developing the classic manifestations of CD. This finding is consistent with that of Beltrão-Teixeira & Carvalho-de Oliveira25 who reported that about 10% of patients with CD-related cardiac complications will eventually develop gastrointestinal manifestations such as megacolon and megaesophagus. However, the intensity of the immune response that occurs in parasite-host interactions is inherent to each patient.
Conclusion
A late diagnosis of CD may increase the chances of presenting digestive complications. However, the classic manifestations of the disease and its comorbidities can be successfully managed as long as comprehensive (multidisciplinary) medical care is provided, since this would help delay the course of the disease and, consequently, improve the patients' quality of life.















