Introduction
According to the World Health Organization (WHO), cancer is one of the leading causes of morbidity and mortality in the world, with 8.8 million deaths reported in 2015.1 Lymphomas are a type of hematological malignancy with highly variable immunophenotypes, clinical and histological features and genetic abnormalities.2 They are classified into two groups: Hodgkin's lymphoma (HL) and non-Hodgkin's lymphoma (NHL),3 and about 500 000 diagnoses of NHL are made worldwide every year.4
As per the 2018 report by the Global Cancer Observatory (GCO), NHL had an incidence of 2.8% with 1 353 273 cases over a 5-year period (2013-2018), while HL had an incidence of 0.44% with 275 947 cases over a 5-year period (2013-2018).5 In Colombia, as reported by the Fondo Colombiano de Enfermedades de Alto Costo (High Cost Diseases Fund), 7 507 and 1 770 patients over the age of 18 were diagnosed with NHL and HL, respectively, between 2015 and 2016.6
Although the clinical presentation of both types of lymphoma is similar, prognosis varies according to the subtype.7 In NHL patients, the relative survival rate is 70% and 60% at 5 and 10 years, respectively,8 while survival in HL patients depends on the stage of the disease: the relative 5-year survival rate is 90% for stages I and II, 80% for stage III, and about 65% for stage IV.9,10 Advances in clinical and therapeutic management of patients with lymphoma, such as autologous and allogeneic stem cell transplants, improve progression-free survival in this disease.11,12
Even though lymphoma patients have specific concerns that generate physical, social, psychological, and functional deterioration,13 to date, in Colombia there are no valid instruments that allow assessing health-related quality of life in this population. Quality of life is considered an outcome of cancer treatment and is used as a measure of well-being in patients with this disease.14 The WHO describes quality of life as "an individual's perception of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards and concerns."15,p3 To assess an individual's quality of life, it is necessary to take into account their subjective perceptions of health, their emotional response to the different situations they face, as well as their level of job satisfaction, their interpersonal relationships and their life purpose.16 With this in mind, the concept of quality of life allows us to obtain information about the impact of the disease and its treatment on both the physical and emotional aspects of patients' lives.
According to the American Society of Clinical Oncology,17 quality of life should be considered a priority outcome of treatment in oncology patients. Therefore, it should be considered for the development of clinical practice guidelines and the evaluation of new technologies for therapeutic purposes.
The scales suggested to measure quality of life in cancer patients include the European Organisation for Research and Treatment of Cancer (EORTC) quality of life questionnaire (EORTC QLQ-C30),18 which is the most widely used instrument in this population,19 and the Quality of Life Patient/Cancer Survivor Version (QOL-CSV) scale, designed based on an instrument to evaluate pain management in cancer patients.20,21 On the other hand, the 36-Item Short Form Health Survey (SF-36), elaborated in the United States, seeks to evaluate the positive and negative states of health, and their Implications in the quality of life of patients with different types of diseases.22,23
One of the few scales that measure quality of life in patients with lymphoma is the Functional Assessment of Cancer Therapy - Lymphoma (FACT-Lym) scale, developed by the Functional Assessment of Chronic Illness Therapy (FACIT) organization in 2005.24 The literature reports on the original validation, carried out in 2013, and the validation carried out the same year in Greece;25 however, the latter is partial as it does not take into account all the clinimetric properties of the instrument. The FACT-Lym scale is made up of the items included the generic module of the FACT-G scale26,27 and by 15 specific items for physical and emotional symptoms of patients with lymphoma. These scales have been designed to be used both in clinical interviews and for self-administration.28
Due to the importance of evaluating quality of life in patients with lymphomas and the unavailability of adequately validated instruments to do so in Colombia, the objective of this study was to establish the clinimetric properties of the FACT-Lym scale in Colombian patients with lymphoma.
Materials and methods
A validation study of scales following the classical test theory was carried out using the FACT-Lym questionnaire. Authorization from the FACIT organization, which was in charge of translating the scale into Spanish, was obtained before beginning with the study. The owners of this instrument do not authorize making new adaptations in Spanish since they already have one in this language, so no cross-cultural adaptation was required.
The sample was selected by convenience and 301 patients who met the following inclusion criteria were included: being >18 years old, verbally agreeing to participate, having a diagnosis of lymphoma at any stage confirmed by histology with or without treatment, and being treated at the Instituto Nacional de Cancerología (National Cancer Institute) between 2016 and 2018 in outpatient, inpatient, or emergency services. Patients with cognitive or sensory impairments that prevent understanding the questionnaire were excluded.
To estimate sample size, the PASS® program was used and all the components of the scale validation process were considered. The exploratory and confirmatory factor analysis was performed on a sample of 301 patients, taking into account the recommendation of MacCallum & Hong29 of having at least 300 sample observations, using the PASS® program and including all the components of the scale validation process. To estimate concurrent validity, a sample size of 163 patients was calculated considering a Pearson's correlation coefficient of 0.2 for the null hypothesis (Ho) and 0.4 for the alternative hypothesis (Ha). To estimate the reliability of the instrument, and using the test-retest method, a sample size of 64 patients was defined, assuming a Lin correlation coefficient of agreement of 0.6 and 0.7 for Ho and Ha, respectively. 30,31 To estimate the internal consistency of the scale, a sample size of 85 patients was established, taking Cronbach's alpha coefficient values of 0.5 and 0.7 for Ho and Ha, respectively, and bearing in mind that the FACT-Lym scale has 15 items.32,33 Finally, in the sensitivity analysis, sensitivity to change was determined based on information from the study conducted by Hlubocky etal.,24 in which, an effect size of -0.87 on the overall score of the FACT-Lym subscale was observed with sample sizes of at least 14 patients.
Description of the questionnaire
The FACT-Lym instrument has two modules: one generic and one specific for lymphomas, consisting of 27 and 15 items, respectively. Its domains are physical well-being (7 items), social/family well-being (7 items), emotional well-being (6 items), functional well-being (7 items) and specific symptoms and concerns related to lymphoma (15 items). Each item has a scoring scale between 0 and 4, with 5 answer options (nothing, a little, some, a lot and very much). These instruments are designed by the FACIT organization in such a way that higher scores indicate a better level of quality of life for the patient in the final score.34,35 The scale was completed by staff trained in its administration.
Statistical analysis
Sociodemographic and clinical data were analyzed by means of descriptive statistics. They are presented using percentages, as well as means and medians with their corresponding standard deviations (SD) and interquartile ranges (IQR). The scale was validated according to classical test theory.
Content validity of the instrument was estimated through an exploratory factorial analysis, which allowed establishing the validity of its construct and, additionally, its domain structure. For the exploratory factorial analysis, the main components of the scale were analyzed based on the polychoric correlation matrix once the possible factorization of the matrix was defined with the Bartlett's test of sphericity and the Kaiser Meyer-Olkin (KMO) test, and the number of factors to be analyzed was determined using the optimal coordinate and the parallel analysis methods.36 To determine the factorial structure, a presence of >0.3 factor loadings was taken into account. The ultimate solution factors were established by applying orthogonal and oblique rotations.37
Confirmatory factor analysis was performed using a polychoric correlation matrix and an asymptotic co-variance matrix. Model fitting was evaluated using the following criteria with the specified values, which indicate a proper model fitting: ratio x2/ degrees of freedom (X2/gL: values <3), root mean square error of approximation (values <0.08), non-standard fit index (values >0.9), goodness of fit index (values >0.9), comparative fit index (values >0.9) and standardized root mean square residual (values <0.08).
The assessment of concurrent validity was performed by calculating Spearman's correlation coefficients38 between the FACT-Lym and FACT-G scale domain scores. The internal consistency of the instrument was analyzed by means of Cronbach's alpha coefficient, which was estimated for the entire scale, for its domains and, also, when each of the items was removed.
To estimate the reliability of the instrument, the test-retest method was used to evaluate the data after administering the questionnaire for the second time to 64 patients, which took place 4 to 10 days after the first time. The data from the second administration were analyzed by means of the Lin's concordance correlation coefficient. Finally, sensitivity to change was measured by comparing the scores obtained before starting the treatment scheme and after its completion or suspension, and was tested using the paired t-test with a two-tailed test and considering a significance level of 5% for hypothesis testing. The statistical analysis procedures were carried out with the R software.
Ethical considerations
The ethical principles for biomedical research established in the Declaration of Helsinki39 were followed. According to Resolution 8430 of 1993 of the Ministry of Social Protection, this study is classified as low risk.40 The study was approved by the Ethics Committee of the Instituto Nacional de Cancerologia through Minutes No. 015 of August 21, 2015.
Results
As for the sociodemographic variables, the average age was 56.7 years (SD=16.4 years), 54.82% were women and 61.4% were classified in the socioeconomic strata 1 and 2 (Table 1). It should be noted that in Colombia, socioeconomic status is classified into strata that range between 1 and 6, being 1 the lowest and 6 the highest (Table 2).
Table 1 Socio-demographic characteristics of the study population.
| Variables | n | % | |
|---|---|---|---|
| Sex | Female | 165 | 54.82 |
| Male | 136 | 45.18 | |
| Marital status | Single | 73 | 24.5 |
| Common-law marriage | 73 | 24.25 | |
| Married | 104 | 34.55 | |
| Divorced | 20 | 6.64 | |
| Widower | 31 | 10.30 | |
| Socioeconomic Stratum | 1 | 73 | 24.25 |
| 2 | 112 | 37.21 | |
| 3 | 100 | 33.22 | |
| 4 | 15 | 4.98 | |
| No data | 1 | 0.33 | |
| Occupation | Home | 92 | 30.56 |
| Unemployed | 66 | 21.93 | |
| Freelancer | 53 | 17.6 | |
| Employed | 48 | 15.95 | |
| Retired | 34 | 11.30 | |
| Student | 8 | 2.66 | |
| Place of origin | Bogotá D.C. | 174 | 57.81 |
| Cundinamarca | 47 | 15.61 | |
| Boyacá | 28 | 9.3 | |
| Tolima | 20 | 6.64 | |
| Atlantic Coast | 11 | 3.65 | |
| Eastern Plains and Amazon | 12 | 3.98 | |
| Other | 9 | 2.99 | |
Source: Own elaboration.
Table 2 Socioeconomic strata in Colombia according to the National Administrative Department of Statistics
| Stratum | Description |
|---|---|
| 1 | Low-low. Beneficiaries of home utility subsidies. |
| 2 | Low. Beneficiaries of home utility subsidies. |
| 3 | Middle-low. Beneficiaries of home utility subsidies. |
| 4 | Middle. They are not beneficiaries of subsidies, nor do they pay surcharges; they pay exactly the amount that the company defines as the cost for providing home utilities. |
| 5 | Middle-high. They pay surcharges (contribution) on the value of home utilities. |
| 6 | High. They pay surcharges (contribution) on the value of home utilities. |
Source: Elaboration based on the data issued by National Administrative Department of Statistics.41
With respect to the clinical variables, 256 patients (85%) were diagnosed with NHL and 45 with HL (14.9%). 19.6% of patients were in stage IVb of the disease, 16% in stage IV, 9% in stage IIIb, 7.67% in stage IIb, and 4% in stage I. Of the total amount of patients, 270 (90%) had received chemotherapy; 47 (15.6%), biological therapy; 22 (7.33%), palliative care; and 19 (6.33%), autologous transplant.
Description of FACT-Lym scale scores and items
After applying the scale scoring algorithm, it was found that the items with the highest scores reached a median of 3. The items that had a lower median and, therefore, influenced a lower level of quality of life were: "I feel pain in certain parts of my body", "I have trouble sleeping at night", "I get tired easily", "I worry about getting infections" and "I am concerned about having new symptoms associated with the disease." Table 3 describes the scores and items on the FACT-Lym scale.
Table 3 Characteristics of the scores and items on the scale.
| ID | Item | Median | IQR |
|---|---|---|---|
| BRM3 | I have episodes of fever that bother me | 3 | 1 |
| LYM1 | I am bothered by the itching | 3 | 1 |
| ES3 | I get night sweats | 3 | 1 |
| P2 | I feel pain in certain parts of my body | 2 | 2 |
| HI8 | I have difficulty concentrating | 3 | 1 |
| C2 | I am losing weight | 3 | 1 |
| GA1 | My appetite has decreased | 3 | 1 |
| LYM2 | I have trouble sleeping at night | 2 | 1 |
| LEU1 | I feel discomfort because of the lumps I have in some parts of my body | 3 | 1 |
| BMT6 | I get tired easily | 2 | 2 |
| N3 | I am worried about getting infections | 2 | 2 |
| LEU6 | I am concerned about having new symptoms associated with my disease | 2 | 2 |
| LEU4 | Because of my illness, it is difficult for me to plan for the future | 3 | 1 |
| BRM9 | I have emotional ups and downs | 3 | 1 |
| LEU7 | I feel isolated from other people because of my illness or treatment | 3 | 1 |
ID: identifier; IQR: interquartile range.
Source: Own elaboration.
Exploratory factor analysis
The results obtained with the Bartlett's test of sphericity (x2 (105) = 1632.935; p<0.005) and the KMO test (0.879) allowed concluding that the matrix had an adequate structure for factor analysis. Given the amount of eigenvalues >1 and the characteristics of the eigenvalue sediment graph, and based on the results obtained with the parallel analysis and optimal coordinate methods , it was concluded that bi-factor analysis was the most suitable to perform the exploratory factor analysis. From the value of each factor loading and according to the interpretability of the different factor solutions, an orthogonal rotation (vari-max) was selected. The uniqueness values were below 0.60. Table 4 presents the factor structure of the sub-scale, in which two domains are differentiated: one of symptoms specific to the disease and one related to patient concerns.
Table 4 Factor structure of the specific subscale (FACT-Lym).
| ID | Item | Factor 1 | Factor 2 | Uniqueness |
|---|---|---|---|---|
| BRM3 | I have episodes of fever that bother me | 0.77 | 0.40 | |
| LYM1 | I am bothered by itching * | 0.75 | 0.43 | |
| ES3 | I get night sweats * | 0.68 | 0.48 | |
| P2 | I feel pain in certain parts of my body * | 0.68 | 0.48 | |
| HI8 | I have difficulty concentrating * | 0.60 | 0.44 | 0.45 |
| C2 | I am losing weight * | 0.60 | 0.43 | 0.46 |
| GAI | My appetite has decreased* | 0.59 | 0.47 | 0.46 |
| LYM2 | I have trouble sleeping at night * | 0.57 | 0.31 | 0.58 |
| LEU1 | I feel discomfort because of the lumps I have in some parts of my body * | 0.55 | 0.31 | 0.59 |
| BMT6 | I get tired easily * | 0.55 | 0.42 | 0.52 |
| N3 | I am worried about getting infections † | 0.87 | 0.24 | |
| LEU6 | I am concerned about having new symptoms associated with my disease † | 0.86 | 0.23 | |
| LEU4 | Because of my illness, it is difficult for me to plan for the future † | 0.38 | 0.72 | 0.34 |
| BRM9 | I have emotional ups and downs † | 0.42 | 0.67 | 0.37 |
| LEU7 | I feel isolated from other people because of my illness or treatment † | 0.47 | 0.53 | 0.49 |
ID: identifier.
* Symptoms of the disease domain.
† Emotional and social component domain.
Source: Own elaboration.
Confirmatory factor analysis
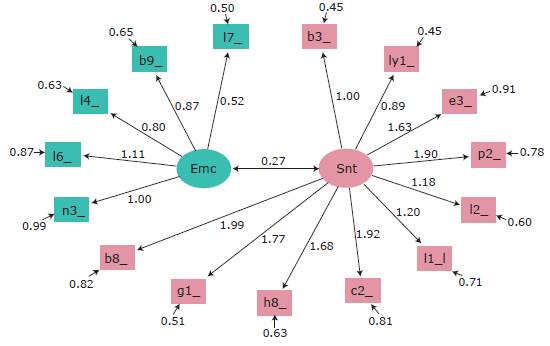
Figure 1 shows the characteristics model for the factorial structure of the FACT-Lym scale. The ovals represent the domains (emotional component and disease symptoms), and the rectangles represent the FACT-Lym scale items (each item is represented with its identifier). Dates marked with a single point indicate the causal relationship between the domain and each of the items, the arrows with double points represent the correlations between domains, and the arrows in dashed lines correspond to loadings that are set with a value of 1 to estimate the coefficients of the models. With respect to the estimators of the equation model, the following results were obtained: x2/gL=0.803; RMSEA=0.000, NNFI=1.010, GFI=0.981; CFI=1 and SRMR=0.062, which showed proper fit of the internal structure of the model.
Concurrent validity
Concurrent validity was assessed in 301 patients based on the scores obtained in the four domains of the FACT-G scale: physical well-being; social/family well-being; emotional well-being; and functional well-being. The values of the correlation coefficients were between 0.24 (correlation with social and family well-being) and 0.73 (physical well-being) (Table 5), so plausible values were considered in the correlation between the four domains of the FACT-G scale and the FACT-Lym subscale. All coefficients were significantly different from 0.
Table 5 Correlation between the four domains of the FACT-G scale and the FACT-Lym subscale.
| Pair correlations | Spearman's correlation coefficient | 95%CI | ||
|---|---|---|---|---|
| Physical | Lymphoma | 0.734 | 0.677 | 0.782 |
| Social | Lymphoma | 0.241 | 0.132 | 0.345 |
| Emotional | Lymphoma | 0.688 | 0.624 | 0.743 |
| Functional | Lymphoma | 0.497 | 0.407 | 0.578 |
Source: Own elaboration.
Internal consistency
The scale had a coefficient a=0.882. The alpha values did not increase when any of the items were removed. Additionally, a coefficient of a=0.8 was found for the mood and/or worry domain and a coefficient of a=0.84 for the fitness domain.
Reliability calculated with the test-retest method
The reliability of the instrument was evaluated in 64 patients, with an average of 7 days between the two evaluations (SD=1.8 days). The medians of the initial evaluation and subsequent measurement were 36 (IQR= 11) and 37 (IQR=9), respectively; the difference between them was not significant (Wilcoxon-signed rank test: Z=-1.12, p=0.26). Lin's concordance correlation coefficient was 0.8 (95%CI: 0.78-0.92). Agreement limits obtained with the Bland-Altman method were between -11.45 and 9.3.
Sensitivity to change
Sensitivity to change was analyzed with data from lymphoma patients between 30 and 60 days after completing or discontinuing treatment, either due to side effects or administrative procedures that did not allow for continuity. For patients treated with allogeneic or autologous stem cell transplants, sensitivity to change was evaluated between 90 and 120 days after the procedure. Table 6 shows the means obtained at each measurement for each domain, which were similar in the two evaluation moments. There were no statistically significant differences (p>0.05 in all paired t-tests performed); this trend is maintained for all subscales developed by the FACIT organization.
Table 6 Comparison of the scores between domains before and after the end or suspension of treatment.
| Domain | Measurement before end or suspension of treatment * Mean (σ) | Measurement after end or suspension of treatment* Mean (σ) |
|---|---|---|
| Physical | 20.46 (4.96) | 20.83 (5.94) |
| Social | 21.7 (5.98) | 19.4 (7.02) |
| Functional | 18.33 (4.67) | 18.46 (3.71) |
| Lymphoma | 30.56 (9.8) | 30.26 (9.34) |
| Emotional | 18.73 (4.05) | 18.46 (3.71) |
* The differences between means before and after the end or suspension of treatment were not significant (p>0.05).
Source: Own elaboration.
Discussion
Health-related quality of life is a concept used to measure the general well-being of patients, which makes it necessary to have valid and reliable instruments to assess the psychosocial aspects of an individual and their influence on health status. Therefore, the FACT-Lym scale was validated in this study to evaluate quality of life in lymphoma patients since it had not been previously done in Colombia.
Most patients included in this study were women, which coincides with the data presented by the Fondo Colombiano de Enfermedades de Alto Costo,6 which reports that the majority of lymphoma cases in the adult Colombian population occur in women. In terms of the clinical characteristics of the patients included, most were in advanced stages of the disease and had received chemotherapy. The most frequent subtype was NHL, which coincides with the data reported by the Global Cancer Observatory.5
Regarding the results obtained in the items of the scale, it has been suggested that the items: "I feel pain in certain parts of my body", "I have trouble sleeping at night", "I get tired easily", "I am worried about getting infections" and "I am concerned about developing new symptoms associated with the disease" may be associated with a lower quality of life in patients and, consequently, represent an important marker of quality of life in this population since the medians obtained for these items were low. However, further studies should be conducted using other methods, such as the Rasch model, to test this hypothesis.
The exploratory factor analysis showed that the FACT-Lym subscale has a simple structure consistent with the FACT-G scale; this analysis allowed establishing two domains: physical condition and specific concerns. Moreover, since the factor loading values were adequate and the uniqueness values were within the recommended ranges, it was concluded that all items in the FACT-Lym subscale are appropriately represented in the factorial structure, suggesting that there were no maladjusted or redundant items in the specific component of the scale.
In terms of internal consistency, the results obtained for the total scale and for each of the items indicated that the FACT-Lym scale has adequate reliability, a result that is consistent with the study by Hlubocky et al.,24 who reported alphas between 0.90, 0.93, and 0.95 on this subscale. With respect to sensitivity to change, no statistically significant differences were observed between the results before and after treatment; however, the differences found in the two measurement moments were very little, and no changes were observed in the other subscales of the FACT-G scale, an instrument that has already been validated and that has adequate sensitivity to change; therefore, it was concluded that the lack of differences is explained by the stability of the construct and not by a clinimetric defect of the scale
Finally, most of the patients included in the study had a low socioeconomic level. This could be a selection bias of the study because perceptions or interpretations related to the socioeconomic situation may have been excluded.