Introduction
Post-traumatic stress disorder (PTSD) is a pathological response to stressful life events that can cause psychological trauma.1 The prevalence of such events in conflicted societies facilitates the appearance of this mental health condition.2 That is the case of Itagüí (Colombia), a city historically affected by violence, where an 11% PTSD rate among the adult population living in its urban area was reported in 2012.3 Besides environmental factors, genetic studies have shown that genetic inheritance plays a vital role in whether this condition is developed after being exposed to a trauma or not. In this sense, a twin study conducted on war veterans showed that genetic factors accounted for 30% of the risk of developing PTSD.4
According to the fear conditioning behavioral models that have been used to explain this pathology,5 PTSD symptoms can be triggered by exposing individuals who have experienced psychological trauma to stimuli associated with said traumatic event. However, in some cases, those associations remain active in time due to the inability of the individuals to inhibit the fear response and, therefore, PTSD symptoms may appear any time over a long period of time, even if the subject is in a safe environment.6 Persistent fear response is thought to be the result of the individual's failure to learn that said emotional response is no longer useful in a safe environment.7
It has been proposed that an individual's genotype can increase the risk of developing PTSD since it can influence how fear is processed in the underlying neural circuit after the subject has undergone a fear conditioning process. Also, this genotype could be associated with prefrontal cortex hypoactivity and hyperactivity in the amygdala;5 thus, it could be involved in how fear memory is consolidated in the corticolimbic pathway.8
SNP polymorphisms in genes that exert a certain degree of influence on different brain pathways have been associated with a fear extinction deficit, including COMT, which codes for the catechol-O-methyltransferase enzyme; BDNF, which codes for the brain-derived neurotrophic factor; CBR1, which codes for the cannabinoid receptor 1; and CCK, which codes for the cholecystokinin neuropeptide.
In the COMT gene, the rs4680 single nucleotide polymorphism (SNP) yields a guanine for adenine substitution, which in turn leads to replacement of valine by methionine in the COMT enzyme, catalyzing the breakdown of catecholamines in the brain. In a fear conditioning experiment conducted on humans, those who had the Met/Met genotype showed a fear inhibition deficit.8 In this regard, Valente et al.9 in a study carried out with Brazilian victims of urban violence, reported that this polymorphism was associated with PTSD. In addition, the Val/Met mutation is also present in the BDNF protein due to a G/A substitution in SNP rs6265 of the BDFN gene. Both mice and humans that carry the Met allele of the BDNF gene have exhibited deficit in fear extinction.10
On the other hand, in the CBR1 gene, rs2180619 SNP can have the A or the G allele. In this regard, Heitland et al.11 reported that the participants with A allele extinguish fear less effectively than those with the G allele. Likewise, the CBR1 gene has also been associated with PTSD.12 Finally, an association between the T allele found in the SNP rs1799923 in the CCK gene and
PTSD was reported in war veterans.13 It has been suggested that cholecystokinin (CCK) neuropeptide acts within the cannabinoid system to modulate fear inhibition; therefore, it is involved in fear processing.14
These findings allow suggesting that the combined effect of polymorphisms in the BDNF, COMT, CBR1 and CCK genes may modulate the risk of developing PTSD after exposure to psychological trauma. In the present case-control study, carried out in the city of Itagüí, an association between PTSD and the aforementioned polymorphisms was tested to determine if these genes may modulate said risk. Both the unilocus and the multilocus effect over the phenotype were considered.
Materials and methods
Previous research
Patient data and genetic samples used in the present study were obtained from a previous mental health survey carried out in 2012 in Itagüí, Colombia.15 For that study, 896 people, living in six urban areas of this municipality, were administered the World Health Organization World Mental Health Composite International Diagnostic Interview (WHO WMH-CIDI). Their saliva samples were collected using the DNA Genotek's saliva-based collection kits. Chelex resins were used for DNA extraction. Finally, the obtained nucleic acids were stored in a Tris-EDTA buffer solution at -20°C.
Ethical considerations
All participants signed and submitted a written informed consent form in which they authorized the use of their DNA samples for future genetic studies on mental health. Data and sample collection procedures complied with the ethical principles for medical research established in the Declaration of Helsinki (2013)16 and the ethical principles for human research stated in Resolution 8430 issued by the Colombian Ministry of Health and Social Protection.17 This study was approved by the Bioethics Committee of Universidad CES on its session 83, held on July 13, 2015.
Participants
Out of the 896 subjects that participated in the study mentioned above, a sample of 129 respondents aged from 13 to 65 years was selected to conduct the present study. All participants from the original sample that had experienced psychological trauma at least once were included in the study. Cases (referred to as PTSD+) consisted of 38 people that were diagnosed with the disorder using the WHO WMH-CIDI. The criteria for diagnosis, according to the interview were: A) exposure to a traumatic event, B) persistently re-experiencing the event, C) persistent avoidance of stimuli associated with the trauma and numbing of general responsiveness, D) hyperarousal, E) experiencing disturbances B, C and D for more than a month, and F) clinically significant distress that impairs normal functioning.18 Controls (referred as to PTSD-) were made up of 91 subjects who only met the diagnostic criterion A.
Sex distribution was similar in both PTSD+ and PTSD-groups (42.1% and 40.6% men, respectively, p>0.5, Fisher's exact test). On the other hand, distribution of comorbidity with major depressive disorder (MDD) was significantly different between cases and controls (52.6% individuals diagnosed with MDD in the PTSD+ group vs. 13.2% in the control group, p<0.001, Fisher's exact test). Finally, a higher prevalence of alcohol dependence was also observed in the cases (15.8% in the PTSD+ group compared to 1.1% in the control group, p<0.005 Fisher's exact test).
Genotyping
SNP rs6265 in the BDNF gene, SNP rs4680 in the COMT gene, SNP rs2180619 in the CBR1 gene, and SNP rs1799923 in the CCK gene were genotyped using kompetitive allele specific PCR (KASPTM) (LGC Genomics, USA). Since it was not possible to find out the genotype of some participants, the following results were obtained: 113 genotypes for SNP rs6265 (33 cases and 80 controls), 107 for SNP rs4680 (32 cases and 75 controls), 109 for SNP rs2180619 (32 cases and 77 controls), and 112 for SNP rs1799923 (33 cases and 79 controls). Selection for double genotyping was blind and random, and only 12.16% of the samples genotyped were selected. The genetic results obtained after this process showed a 100% match between each sample and its replica. Negative controls were also processed blindly and, as expected, it was not possible to genotype them.
Statistical analysis
Statistical data processing was done using the R software version 3.2.2.19 The SNPassoc package20 was used to perform the Hardy-Weinberg test and the unilocus and multilocus analyses. The Epitools package21 was used to carry out a post-hoc analysis to study the allele combinations that could explain gene-gene interactions. All alleles were found in the study population with a frequency greater than 1%. In addition, the Hardy-Weinberg Exact Test22 was used to evaluate the adjustment of the allele frequencies to the Hardy-Weinberg Model, which allowed determining that the allele frequencies of the four genes were in equilibrium.
The unilocus association with PTSD was assessed for each of the candidate genes. The Odds Ratio (OR) was calculated as a measure of risk, while significance was assessed with a maximum likelihood test. The Akaike information criterion (AIC) was used to judge which inheritance model (codominant, dominant, recessive, and over-dominant) fit the data better. The multilocus genetic association between every possible combination of SNP pairs was evaluated as an interaction in the logistic regression model. Age and sex were controlled in the unilocus and multilocus tests. Afterwards, Fisher's exact tests were performed to determine which genotypic combinations of the previously found interactions were significant as risk factors. This was made by comparing each genotypic combination to the rest of the existent genotypic combinations in the sample. Significance tests are reported with a 95% confidence interval.
Results
Unilocus analysis
Only the inheritance models that showed the best fit for the data (smallest AIC) were considered for the unilocus analysis. These inheritance models allowed determining the possible risk genotype for each independent gene. No significant association with post-traumatic stress disorder was reported in any SNP. However, OR intervals tended to be displaced to the right for the following genotypes: AG in the BDNF gene, AA in the COMT gene, and the TC in CCK gene (Table 1).
Table 1 Candidate genes as unilocus risk factors in PTSD cases.
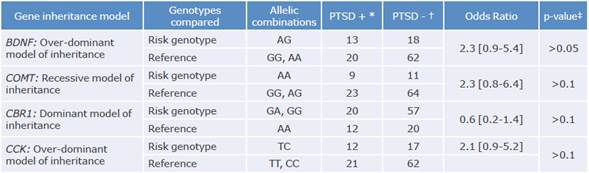
BDNF: brain-derived neurotrophic factor gene; COMT: catechol-O-methyltransferase; CBR1: cannabinoid receptor 1; CCK: cholecystokinin.
*Number of cases diagnosed with the disease.
†Number of controls.
‡ Probability.
Source: Own elaboration.
Multilocus analysis
When considering all possible combinations of SNP pairs in the regression model, interactions between BDNF and CBR1 (p<0.05) and between CCK and COMT (p<0.005) were significant in the PTSD+ group. Then, existent genotype combinations for these interactions were studied individually as risk factors and the following outcomes were obtained: when AG genotype of BDNF gene is combined with AA of CBR1, and when TC of CCK is combined with AA of COMT, the risk for developing PTSD increases significantly in comparison to other existent combinations (Table 2).
Table 2 Genotype pairs of SNP-SNP interactions identified as risk factors in PTSD cases.
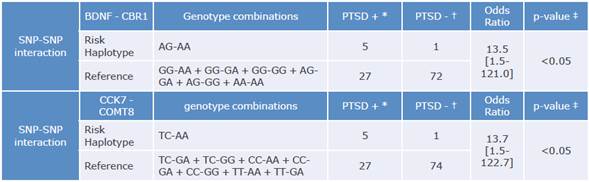
SNP: Single nucleotide polymorphism; BDNF: brain-derived neurotrophic factor gene; CBR1: cannabinoid receptor 1; COMT: catechol-O-methyltransferase; CCK: cholecystokinin.
* Number of cases diagnosed with PTSD. t Number of controls.
* Probability.
Source: Own elaboration based on the data obtained in the study.
Discussion
Unilocus vs. multilocus results
According to the reports, risk allele A of the BDNF, CBR1 and COMT genes and risk allele T of the CCK gene have been associated with increased vulnerability for PTSD9,13 or reduced capacity to inhibit the fear response.8,10,11 However, in the present study, no association between carrying these alleles, either in a homozygous or a heterozygous form, and developing PTSD as a result of being exposed to a traumatic event was found.
Nevertheless, when these alleles were portrayed in two different genes, they behaved as risk factors for developing the disease. This was the case for A allele as a heterozygous in genotype AG of BDNF combined with A allele as a homozygous in genotype AA of CBR1, and for T allele as a heterozygous in genotype TC of CCK combined with A allele as a homozygous in genotype AA of COMT. The low frequencies of genotype AA of BDNF (0.7%) and genotype TT of CCK (2.8%) that were observed in this study might explain why this effect is only present when these alleles appear as heterozygous. Thus, portraying two risk alleles in two different interacting genes increases the risk of developing PTSD after exposure to trauma.
SNP-SNP interactions associated with posttraumatic stress disorder
BDNF interacts with the endocannabinoid system inducing the synthesis and release of neuromodulators (endocannabinoids and neurotrophins). Once they are released, they activate their CBR1 receptor, starting a long term depression of inhibitory synapses.23 It is known that the substitution of valine for methionine in the BDNF precursor protein affects the depolarization dependent-secretion of this growing factor in hippocampal neurons.24 If BDNF is secreted in a less effective way, its activity on the endocannabinoid system may be affected; this system plays important role in the expression and retention of fear extinction.25 A minor activity of BDNF, together with portraying AA genotype in the SNP rs2180619 of the CBR1 gene might explain why an association between these two interacting genes and PTSD was found in the present study.
Regarding the interaction between the gene products of CCK and COMT, it is known that the CCK neuropeptide exerts antagonistic effects on dopaminergic function through its action on the CCK-B receptor.26 The CCK polymorphism under study appears to reduce the transcription of this gene.27 Meanwhile, when the COMT enzyme is mutated with Met allele, its catalytic activity drops by 40%, increasing dopamine availability in the brain.28 These two effects combined may impact the antagonistic effects of CCK over dopaminergic signaling, where the antagonistic effect of CCK is important to modulate behavior motivated by stress.26 Yet, determining how a reduction of this modulatory action could be associated with PTSD requires further research.
Limitations
One of the limitations of this study is that the frequency of comorbidity with MDD was higher in the cases than in the controls. As a result, the genetic associations found here may be a consequence of an MDD diagnosis instead of a PTSD diagnosis. However, it is well known that comorbidity between MDD and PTSD is usually the standard. Depressive symptoms appear along with PTSD symptoms, and some authors consider that separating MDD into a different disorder when it co-occurs with PTSD is arbitrary.29 Consequently, both diseases could share the genetic factors that increase the risk of developing them after an individual is exposed to a traumatic event.
Another limitation of this study is that the sample of PTSD patients retrieved from the previous study was low since only 38 people were diagnosed with the disorder out of 896 people that were originally interviewed. Thus, the presented results may need additional support by further studies.
Conclusions
This study shows that the probability of developing PTSD is higher when two risk alleles are present in two interacting genes than when the allele is only found in one of the two genes. This means that to understand the genetic etiology of this disease better, the effect of multiple polymorphisms acting together on the phenotype should be considered. It is worth noting that the interactions found here should be analyzed at a biochemical level to understand how they influence the neural networks in which fear processing occurs and establish a causal relation between said influence and PTSD. Although the way how these genes interact at the neuronal level is not clearly known, it was found that certain genetic combinations, considered as risk factors, could be used to help achieving a genetic diagnosis of the disease.















