Introduction
The Asociación Colombiana de Medicina del Sueño (Colombian Association of Sleep Medicine - ACMES) was established in 1998 with the aim of spreading this medical specialty in the country.1 Since then, the number of sleep laboratories has increased, and the creation of specialized centers has been encouraged not only to conduct studies but also to comprehensively treat patients with sleep disorders, which implies having an interdisciplinary team available for that purpose.
Due to the rapid spread of the new coronavirus (SARS-CoV-2), which is the source of the current COVID-19 pandemic, most sleep units restricted and/ or suspended the provision of their services to prevent contagion. However, this situation cannot be indefinite because patients need care. Therefore, these specialized centers must resume their operation following all biosafety protocols to protect the health of both patients and staff.
Since there is a possibility that selective isolation and contingency measures may be prolonged until an effective vaccine is available in Colombia, the ACMES proposed a series of recommendations to develop activities related to sleep medicine during the pandemic, protecting the health of all actors involved and, in this way, carry out all pending procedures. These recommendations are based on the mitigation strategies established by the country's health authorities and the literature available on the subject, which was reviewed after conducting a search in the PubMed, SciELO and Google Scholar databases using the terms "sleep," "sleep medicine" and "COVID19".
Sleep overview
Sleep is essential for human life and it has undergone changes with the evolution of the species.2 It is also a highly complex process and new aspects are discovered everyday through studies on its physiology, biochemistry, genetics, synchronization with environmental stimuli and related alterations, which have a significant impact on people's lives.
The nervous system is fundamental in the sleep-wake cycle because the brain goes through three different phases during this process: wakefulness, slow-wave sleep (non-REM) and paradoxical sleep (REM). These phases are regulated by circadian rhythms and the suprachiasmatic nucleus, where light and environmental stimuli help sleep processes function properly.3
Until a few centuries ago, medicine only studied the human being in wakefulness, and, therefore, very little was known about the functioning of the body during sleep. Currently, this situation has changed to the point of having a specialty called sleep medicine and having an international classification that, in its third edition, has 8 chapters with clinical criteria to recognize disorders related to the sleep process.4
There is no doubt that the lack of sleep, and its reduction or poor quality, affect wakefulness and alters the function of different organs. So, to make an effective and timely diagnosis of a sleep disorder and provide the patient with a good treatment, the clinical approach to these cases should be comprehensive, including, in many cases, testing in specialized laboratories.
Sleep studies
For their operation, sleep units must comply with local health standards and, when applicable, they should be certified by the competent authorities.5 The most frequent studies performed in these institutions are baseline polysomnography (PSG) with oximetry, PSG with titration -CPAP (continuous positive airway pressure), BiPAP (bilevel positive airway pressure), adaptive servo-ventilation, high-flow nasal cannula, mandibular advancement device, and postural device for obstructive sleep apnea/ hypopnea syndrome (OSAHS)- split-night PSG, multiple sleep latency tests, maintenance of wakefulness test, nocturnal respiratory polygraphy, actigraphy, capnography, and restless legs test.
PSG is a study frequently performed that causes minimal discomfort to patients and does not cause pain. In this study, techniques such as electroencephalography, electrooculography, and chin and lower limb electromyography monitor the variables snoring, respiratory effort and flow, heart rate, blood oxygen levels, body position, and others, according to the needs of each patient. There are many PSG modalities, but the most common is split-night PSG with monitored oximetry.6
Sleep studies are useful to diagnose different sleep disorders, with SAHOS being the most frequently detected.
The COVID-19 pandemic
A pneumonia outbreak was reported in December 2019, in Wuhan, China, and on January 7, 2020, the causative agent was established as a novel group 2B coronavirus (SARS-CoV-2) of the same family as the SARS.7 On February 11, 2020, the World Health Organization (WHO)8 called the disease caused by this virus COVID-19, and a month later, because of its rapid spread and its presence on all continents, it declared it a pandemic.9
In Colombia, the first COVID-19 case was reported on March 6, 2020, in a woman who arrived in the country from Milan, Italy. In the following days, more imported cases were detected, which were accompanied by household contact cases. At present, the country is at the community level phase of the outbreak, which is growing despite the recommendations from local authorities, namely, frequent handwashing, use of face masks, social distancing, and selective lockdown.10-12
In this context, multiple research works on this virus and the disease it causes have been carried out worldwide. Studies have been oriented toward decision-making regarding the management of the situation and the treatment of the disease, and they vary rapidly based on new scientific findings and possible new virus behaviors. For example, based on the experiences of countries like the United States, Spain, Italy, France, Germany, the United Kingdom, and Ecuador, where health systems collapsed in several of their cities, and there were real situations of widespread emergency,13 rapid and timely actions have been taken in Colombia to adapt health services to cope with the imminent emergency. Some of these measures include canceling elective surgeries, restricting the use of anesthesia machines and surgical ventilators to treat possible cases of COVID-19, and suspending outpatient care services.10,14
In this regard, the American Academy of Sleep Medicine (AASM) recommended that diagnostic PSGs should not be performed because this procedure may contribute to the contamination of patients or caregivers through fomites or devices that come into close contact with the patient, including cannulas, electrodes, and sensors. Specifically, the Academy advised against performing CPAP titration PSG due to the risk of aerosolization of the virus.15,16
Telemedicine
Telemedicine (TM) is the provision of remote medical services using communication and information technologies. It is focused on caring for people who, due to multiple situations, cannot access face-to-face consultation. Under the current circumstances of the COVID-19 pandemic, TM is a care modality that can prevent patients from being exposed to hospital settings and thus reduce the risk of infection.17 It is also a care strategy by which health professionals can reduce their exposure to sick patients who may transmit the virus.18
Ohannessian et al.19 state that video calls helped reduce the risk of COVID-19 contagion in the United Kingdom, the United States, China, and Australia. For this reason, the implementation of TM is considered a good option to treat patients safely and effectively amid the current situation.20,21
According to the International Labour Organization (ILO), the crisis generated by the COVID-19 pandemic and the consequent massive disruption of economic activities affect all trades.22 In particular, the negative impact on health workers is mainly associated with their close contact with the virus, which puts them at a higher risk of infection. In such a scenario, TM arises as a safer modality for health care service delivery. In Colombia, this modality is legally supported by Law 1419 of 2010,23 and resolutions 5857 of 2018,24 2654 of 20 1 925 and 2003 of 2014,26 issued by the Ministry of Health and Social Protection (Minsalud).
Teleorientation, telediagnosis, teleconsultation, tele-expertise, teletherapy and telemonitoring are the forms of TM used in sleep medicine.27 In particular, teleconsultation has proven to be useful in the follow-up and treatment of patients with OSAH.28-30
To implement TM, patient safety and good care must be ensured. To this end, it is necessary to count on responsible and competent professionals who can report as much patient information as possible in the medical records.31 It is also recommended to inform in the medical record that the consultation was remote due to the COVID-19 pandemic.
Below is a proposal of what should be considered for teleconsultation in the country taking into account the current contingency:32-34
The health insurer must expressly authorize this type of consultation to facilitate the procedures to the user (picking up prescriptions, results of procedures and medical orders).
Fees must be billed online before the teleconsultation begins.
Patients, or parents/guardians, if the patient is a child, must be asked for their authorization (informed consent) to perform this type of consultation; this authorization must be registered in the medical record.
The patient's availability to attend consultation should be confirmed by phone the day before the appointment and ideally 15 minutes prior to the appointment as well.
The teleconsultation must start once the patient joins the online session.
The consultation must follow the parameters established by the regulations.
A detailed care record should be included in the medical record indicating that no complete physical examination was performed due to the nature of the consultation.
Prescriptions and medical orders should be generated as in face-to-face consultations.
Prescriptions, medical orders, and medical records should be emailed to patients.
Finally, it is advised to follow up on the patient's clinical condition, if required, and the orders issued.
Outpatient service
Patient care in the outpatient service is key for clinical follow-up of different sleep disorders, which may increase during the COVID-19 pandemic.33 In order to deliver this service, the health care provider must implement isolation strategies for patients with respiratory symptoms. The recommendation is to carry out a triage by telephone the day before the appointment to detect symptoms of COVID-19 and install a triage station at the entrance of the service to measure temperature and evaluate the patients' condition the same day of the appointment before they enter the facilities. This strategy will allow detecting patients with respiratory symptoms and isolating them immediately.
Similarly, it is suggested to follow Minsalud's indications for outpatient services, which are summarized below:35,36
Adopting social distancing measures to ensure a minimum space of two meters between each person.
Implementing barriers to protect health personnel.
Providing health care personnel with the personal protective equipment (PPE) necessary to carry out their work safely (surgical masks, gloves, water, soap, etc.) and training them on the proper use of these items.
Having special settings available to treat patients with respiratory symptoms.
Equipping the facilities with the necessary supplies for hand hygiene and verifying that this practice is done correctly.
Ensuring cleaning and disinfection of surfaces.
Other important recommendations are to schedule appointments every 30-45 minutes to avoid crowds in the waiting room; ensure punctuality; allow only one companion per patient, who must not have respiratory symptoms or be an adult over 60 years of age; request the patient and their companion to use face masks; remove items that can facilitate cross-contamination from common areas, such as magazines, brochures and toys; and maintain proper ventilation at the doctor's office and the waiting room.37
For the health staff, the recommendation is to maintain standard biosafety measures in health service delivery institutions such as:
Using surgical masks.
Using gloves only for procedures with secretions.
Washing hands according to the WHO Five Moments for Hand Hygiene approach: before touching the patient, before a procedure, after a procedure or being exposed to body fluids, after touching the patient, and after touching the patient's surroundings.
Prioritizing care of patients with suspected severe acute respiratory infection (SARI) and putting them in quarantine to avoid contact with others.
Sending patients with respiratory symptoms back to their homes or instructing them to go to the emergency department, if necessary.
Promoting cleaning and disinfection of contaminated materials and surfaces.
Similarly, it is necessary to provide information to patients and accompanying persons on care to prevent SARIs and information on the initial management of symptoms at home and signs of alarm that require medical advice.31,38,39 Information provided to the patient should always be registered in the medical record, not only through the document that is institutionally used for this purpose but also through notes; this will reduce the risk of loss of information.
Specifically, sleep medicine professionals should not only make the usual notes established by the institution, which include mandatory reporting of infectious diseases,40 but they also must investigate the symptoms and signs of sleep disorder that generate the consultation to provide the necessary guidance in each case and establish the plan to follow.
Outpatient studies
At-home testing for OSAHS is an alternative to PSG when it is necessary to diagnose adult patients with high suspicion of the syndrome. However, this test has some limitations, as it cannot be used as a screening test or replace the gold standard, and the results must be interpreted carefully in the light of the AASM recommendations.15,16 In summary, this test is a useful and practical alternative that can reduce the risk of contagion for the patient and the sleep unit staff during the current public health emergency only if the parameters for safe operation are followed.
It should be noted that at-home testing is indicated for people with high suspicion of OSAHS who have neither moderate to severe complications nor are vulnerable to COVID-19,15,16,41,42 and that the following aspects must be considered:
Disposable devices and/or electrodes should be used.
If reusable devices are used, they should be cleaned and disinfected individually following the Communicable Disease Center's disinfection and sterilization guidelines.15
It must be ensured that patients do not leave their homes to receive or return the device (polygraph/polysomnograph), which requires hiring a courier company.15 In case it is not possible to use the mail service, a relative who is not in the high-risk group should collect and deliver the device to the sleep unit, following the recommendations for the prevention of infections.29
Patients need access to instructional brochures and TM consultations to ensure proper device configuration, proper placement of electrodes or recording device, and safe operation of the device when they receive and return it.15
Once the test is complete, the patient must be asked to discard the cannulas at home. If there is risk of damage to the connection inlet of the device, the cannula must be cut at 10cm from the end inserted in the device and the sleep technician who receives the device should remove the remaining cannula.29,41
The device should be returned in a closed bag or in a heavy-duty plastic box with lid and handle.41
It is necessary to ensure that persons receiving and cleaning the devices and/or electrodes use the appropriate PPE and follow the manufacturers' cleaning instructions.15,16
The device must be handled at least 72 hours after being returned.16
The patient's study information must be extracted using the appropriate PPE.16,29
Sleep unit studies
Recommendations for performing sleep studies should be evaluated by the providers of these services based on international standards to minimize exposure to the virus for all the people involved.16,29 In addition, studies done using positive airway pressure (PAP), such as CPAP titration or split-night studies, should be postponed for as long as possible due to the risk of aerosolization. In the event that these procedures cannot be postponed, it is necessary to use all protective measures, including the use of full PPE, and consider the use of non-invasive ventilation masks and antiviral filters; the adaptation process must be carried out with the equipment turned off.43-45
Similarly, it is necessary to try to postpone diagnostic PSGs in children, adults over the age of 70, pregnant women, and patients with significant medical comorbidities such as diabetes, heart and lung disease, or any condition that compromises the immune system. When PSGs cannot be postponed and need to be performed, the following aspects should be considered:
Health and administrative staff who have contact with the patient (secretaries, receptionists, or general service personnel) should be trained in key concepts of the disease, such as etiological agent, virulence, means of contagion, personal protective measures, personal hygiene measures (especially handwashing) and use of face masks according to the level of risk (how to put them on and take them off and their disposal).
Patients who are scheduled for sleep studies should be asked by telephone about symptoms and risk factors when confirming assistance to the procedure. If they have symptoms or if they have had contact with someone diagnosed with COVID-19, they will be rescheduled and will be informed about preventive isolation measures, hygiene standards and the hotline enabled to follow-up suspected cases (Table 1, Figure 1).
Patients who need to be accompanied must be rescheduled. If priority is given to conducting the study, the companion should also be asked about the presence of symptoms and risk factors.
Technical personnel conducting the procedure should be frequently asked about symptoms or risk factors. Those presenting with fever, persistent dry cough, dyspnea, and anosmia, or who have had direct contact with confirmed or suspected cases of COVID-19 should take preventive isolation measures and contact the appropriate follow-up hotline.
Technical personnel must receive the required PPE: surgical clothing, N95 face masks, protective gloves and goggles, or protective masks for use while in the same room with the patient.
Both the patient and the sleep unit staff must follow general biosafety recommendations, including temperature measurement with infrared thermometer and handwashing before entering the facilities, before and after entering each room, and before having contact with the patient or handling the devices. They must also wear their hair up; have short nails without nail polish; not wear rings, earrings, or chains; not touch their face; and cover their mouth with their forearm when coughing or sneezing.
Institutions must have glycerin gel available inside and outside the room.
All supplies should be disposable, but if this is not possible, they should be cleaned and disinfected according to the manufacturer's instructions and the institution's protocol.
The contact surfaces of the unit (tables, door handles, keys, water taps, toilets, telephones, keyboards, beds, etc.) must be cleaned and, if applicable, disinfected before each patient enters and leaves.
The rooms should be ventilated and disinfected according to the institution's protocol after the patient leaves.
The specialist physician should interpret the results of the study remotely.
Table 1 Preliminary questionnaire.
| Question | Yes | No |
|---|---|---|
| Have you traveled abroad in the past 15 days? | ||
| Are you a health worker, or do you hold a position in a hospital that may require you to have close contact with probable or confirmed cases of people with COVID-19? | ||
| Do you have a history of close contact with a probable or confirmed case of severe acute respiratory infection associated with COVID-19 within the last 14 days? | ||
| Have you presented with at least two of the following symptoms: fever higher than 38°, cough, dyspnea, odinophagia, fatigue/adynamia, decreased sense of smell (hyposmia) and/or distortion of the sense of taste (dysgeusia)? |
Source: Own elaboration.
Cleaning, disinfection, and storage
According to the review by Kampf et al.,46 endemic human coronavirus (HCoV) may persist on inanimate surfaces such as metal, glass, plastic, paper, and wood from 4 hours to 9 days; time varies depending on the surface type, humidity, and room temperature: at higher temperatures, less surface persistence.46 The stability of SARS-CoV-2 is very similar to that of HCoV-19 and SARS-CoV-1 viruses, that is, they can remain viable in aerosols for 3 hours, in cartons for up to 24 hours, and in plastic and stainless steel for up to 3 days. In these circumstances, it is suggested to comply with the following general recommendations regarding cleaning, disinfection, and storage of items in contact with patients and workers in sleep units:
Cleaning surfaces, first, with a mixture of water and standard detergent, then rinse them with pure water.
Disinfecting the surfaces with a dilution of 0.5% sodium hypochlorite (1:100 dilution of 0.5% sodium hypochlorite) or a mixture of 1 tablet of sodium dichloroisocyanurate dissolved in 1 liter of water.
Disinfecting reusable specialized devices with 62-71% ethyl alcohol.
Using hydrogen peroxide-based products, such as Ox-ivir, or fourth generation quaternary ammonia-based products that clean and disinfect at the same time.
Cleaningrespiratory polygraphs, polysomnography devices, and PAP therapy machines
Cleaning of devices used in sleep studies should be limited to external surfaces and should be done upon receiving the device and before delivering it to another patient if it was not kept in a protected environment. A cloth moistened with enzymatic detergent or disinfectant detergent should be used for this procedure, and the surfaces should be thoroughly cleaned, avoiding touching electrical connection sites. Then, with another cloth moistened only with clean water, it is necessary to go over the surfaces and allow them to dry. For PAP devices, if applicable, the recommendation is to wash the filter with soap and water or hospital detergent after each study. The pulse oximeter sensor of the respiratory polygraphs and polysomnography devices must be cleaned with a cloth moistened with soap or disinfectant detergent. Abrasive agents, alcohol, acetone, or substances containing chlorine, solvents or glutaraldehyde should never be used for cleaning these devices, and saline solution rinses are not recommended.
Specifically, for disinfection, a surface disinfectant should be applied with a clean disposable cloth and left to work for approximately 5 minutes. Then, it is necessary to wipe the device with another clean, dry disposable cloth to remove any residual disinfectant.
Cleaning electrodes, thermistors, and other elements
Cup electrodes, also known as surface electrodes, are first cleaned as usual by rubbing them with a bristle brush moistened with water only and leaving them to air-dry. Then, if the parts are suitable for immersion, they should be left for 5 minutes in enzymatic detergent, and if they are not, they should be sprayed with detergent-disinfectant.
Thermistors must first be sprayed or immersed in Surgizime for 15 minutes to clean, and then placed in Cidex OPA for 12 minutes to disinfect and left to dry completely.
For cleaning the respiratory belts, it is suggested to consult the manual of each brand of respiratory polygraphs and polysomnography devices. If they cannot be cleaned, the recommendation is to cover them with washable or disposable material, such as plastic tubular film, between studies. If they are washable, they should be cleaned with warm soapy water or disinfectant cleaner. It is necessary to ensure that the belts and connectors are completely dry.
Storage
All non-disposable items used in the different procedures carried out in sleep units should be stored in dry, non-dusty places, and temperature should be maintained in a range of 20°C to 60°C. They can also be stored individually in containers so that they are kept sterile and/ or disinfected until their next use.
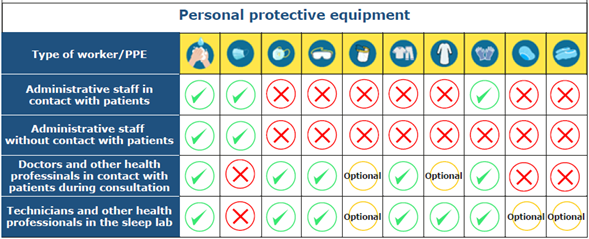
Personal Protective Equipment (PPE)
All personnel attending sleep clinics (patients, companions, and workers) must wear PPE.
PPE for administrative staff in contact with the patient
People who work as receptionists or secretaries and who are not part of the health care staff should have a work-only suit, wear conventional face masks and non-sterile gloves, wash their hands constantly, and maintain social distancing. The keyboard of the computers used by these people should be covered with a disposable plastic protector that needs to be changed daily.
PPE for administrative staff without contact with the patient
Administrative staff who do not have contact with patients should only use conventional face masks, follow social distancing guidelines, and wash their hands frequently.
PPE for doctors and other health professionals in contact with patients during consultation
The sleep medicine clinic is at intermediate risk of COVID-19 infection, which is why somnologists must wear a work-only suit and a N95, N99, FFP2, or FFP3 mask, which, depending on the quality of the material, can be reused after rigorous disinfection or sterilization. These professionals should not wear any makeup or jewelry, and men should not grow a beard. The use of safety goggles is optional, but they are recommended if the oral cavity must be examined.
PPE for technicians and other health professionals in contact with the patient in the sleep lab
Sleep studies are considered to be a high-risk factor for COVID-19 contagion, so technicians in charge of performing these studies should, on the one hand, use safety goggles, ideally with face seal, and an N95, N99, FFP2 or FFP3 mask, and, on the other, wear a work-only suit and an anti-fluid overall or gown on top of the suit, which may be reused after rigorous disinfection or sterilization depending on the quality of the material. Technicians should not wear any makeup or jewelry, and men should not grow a beard. Although the use of hats and shoe covers is optional, it becomes mandatory in case they use PAP equipment given the aerosolization rate. Double gloving is indicated so that the technician can use the external gloves during the patients' initial management and discard the PPE with the internal ones after the procedure is completed.
External PPE such as gloves, face masks and overalls should be replaced each time a patient is treated with PAP devices.
PPE disposal
Items that are not reusable must be placed in red bags and comply with the established sanitary disposal route for hazardous waste. It is necessary that the person in charge of handling these wastes wears the same type of PPE used by the technicians in charge of performing sleep studies (Figure 2).
Recommendations to patients on PAP therapy
The CPAP and BiPAP positive pressure devices generate aerosols47 that can transmit directly the SARS-CoV-2 virus47 or contribute to surface contamination.48
In patients with OSAHS on PAP therapy without clinical suspicion or symptoms of COVID-19, the equipment may be used if the mask, circuit, and equipment cleaning recommendations are strictly followed.49 At the same time, it is suggested to evaluate the risks and benefits of continuing to use the CPAP or BiPAP devices in ambulatory patients with COVID-19. It should be noted that although OSAHS is a chronic disorder, the risk of stopping PAP therapy for a limited period until the patient is no longer contagious can be manageable depending on the severity of the disorder and the symptoms.
Risks of continuing treatment
If PAP therapy is continued, the risk of virus transmission in individuals residing near the patient may increase, especially in those at risk for severe disease. In addition, it has not been determined whether reinfection by the reuse of probes and mask filters is possible.
Risks of suspending treatment
Stopping CPAP therapy may increase traffic and work-related accidents, falls, or cardiovascular events in some patients and may make symptoms such as daytime sleepiness, lack of concentration, and tiredness return.
Also, it should be noted that non-invasive ventilation suspension in patients with chronic hypercapnic respiratory failure may worsen symptoms such as dyspnea and headache and may accelerate acute hypercapnic respiratory failure.50
With these risks in mind, alternatives such as positional therapy or the use of mandibular advancement devices (if the patient already has one) should be considered when stopping PAP therapy; similarly, alcohol and sedative drugs should be suspended, patients should stop driving, and measures to prevent falls should be implemented.
Moreover, if the use of PAP therapy in suspected or confirmed COVID-19 patients is strictly necessary, it is recommended to keep these people in strict quarantine and implement strategies to protect other residents of the household. In addition, the cleaning and disinfection protocol for the devices, masks and tubes recommended by the manufacturers must be followed; these procedures must be done by the patient to prevent other people from handling them.
CPAP should not be shared for any reason, and leaks should always be overseen as they increase particle dispersion. It is recommended to have the humidifier switched off.
In hospital patients with confirmed COVID-19, CPAP should be avoided, so other measures, such as raising the head of the bed, initiating nasal cannula, and limiting airway manipulation and procedures that may increase virus dispersion, should be considered. If used, strategies must be designed to protect health care professionals and other patients.15
Pediatric care
According to data collected from COVID-19 cases in China by Zimmermann & Curtis51, children usually present with mild symptoms. In addition, as stated by Wu & McGongan,52 the number of affected children is lower compared to adults: of the 72 314 cases reported as of February 2020 in that country, only 2% were under 19 years of age.
In the study by Castagnoli et al.,53 none of the children diagnosed with COVID-19 had comorbidities, 65% had mild to moderate symptoms, and only 9% were asymptomatic. The latter finding contrasts with that reported for adults, where asymptomatic patients account for 70% of infections. Castagnoli et al.53 also proposed that children may be a reservoir or a population at risk for infection, but in that study, most children contracted COVID-19 in a family outbreak, in which symptoms appeared in 82% to 100% of cases after another family member acquired the disease. This suggests that the infection originated in the household and that this population is not at increased risk of being a source of infection to others.53
Although the AASM does not recommend pediatric sleep studies, different studies show that children are not the index case, nor are they an important repertoire of infection.54-56 Therefore, given the scarce evidence available to date and the increase in sleep disorders among the pediatric population during the mandatory lockdown, performing sleep studies in this population could be considered individually and measuring the cost-benefit ratio.
All the recommendations presented here are relevant since, in Colombia, especially in Bogotá through Circular 029 of April 29, 2020, issued by the Mayor's Office,57 surgical and/or diagnostic procedures postponed during the containment phase of the emergency must be resumed.
Conclusions
This reflection, which is based on the mitigation strategies suggested by the Centers for Disease Control and Prevention included in the latest AAMS update of recommendations relevant for reopening sleep practices during COVID-19,15 shows that Colombia is implementing the necessary strategies to reduce the impact of the current COVID-19 pandemic and proposes a series of recommendations for somnologists and local sleep units to resume their activities and guide their practice during this contingency. These recommendations are expected to comply with national and international guidelines to reduce the risk of infection and to guarantee access for patients with sleep disorders to the comprehensive diagnosis and treatment of their disease.