Introduction
Acute promyelocytic leukemia (APL) is a subtype of acute myeloid leukemia (AML) with unique morphological, cytogenetic, and molecular characteristics. In the United States, 4-8% of children with AML have APL,1 and in Latin America, a higher incidence has been reported in Brazil (28%),2 Mexico (20%), and Venezuela and Peru (22% each).1-3
APL is characterized by a reciprocal translocation between the PML gene on chromosome 15 and the retinoic acid receptor alpha on chromosome 17, which forms the PML-RARa protein.1,2 This translocation -t(15;17)- is found in 95% of cases of this type of leukemia; however, APL can also be caused by other translocation variants such as t(5;17) and t(11;17) that cause 5% of cases and have similar pathophysiological effects.2,4
Severe coagulopathy and bleeding are the main complications of APL, which are more common in the microgranular variant (M3v). Since 1980, with the discovery of all-trans retinoic acid (ATRA) -a drug that induces the differentiation of promyelocytes into mature granulocytes-, a new era in the treatment of this disease began, in which a marked increase in both overall survival (OS) and event-free survival (EFS) has been achieved.1
The PETHEMA LPA 99 protocol was developed by the Spanish Society of Hematology and Hemotherapy within the framework of the Spanish Program for the Treatment of Malignant Hemopathies to treat APL. It has shown favorable EFS and OS rates with acceptable toxicity in the induction, consolidation, and maintenance phases in adult patients. This protocol has criteria for the classification of disease risk and uses ATRA in all phases of treatment: combined with anthracyclines during induction-consolidation, and with mercaptopurine and methotrexate during maintenance until completing 2 years of treatment. The implementation of this strategy has achieved an OS >80%.
Countries such as Spain, Netherlands, Belgium, Czech Republic, Japan, Korea, Turkey, Tunisia, Argentina, Brazil, Chile, Mexico and Uruguay have achieved good survival results with the implementation of the PETHEMA LPA 99 protocol, as reported in the literature.5-12 In Colombia, in 2011, an evidence-based clinical practice guideline for the care of children with APL was developed, which included the same drugs that are recommended in the protocol as treatment drugs.13
The main objective of the present study was to describe the results of the PETHEMA LPA 99 protocol implementation in the treatment of children with APL at the Fundación Hospital Pediátrico La Misericordia, located in Bogotá D.C., Colombia.
Materials and methods
A descriptive and retrospective cohort study was conducted. The medical records of 30 pediatric patients (under 18 years of age) with APL who received treatment based on the PETHEMA LPA 99 protocol at the Fundación Hospital Pediátrico La Misericordia between January 2005 and December 2012 were reviewed. Data on the following variables were obtained: early death, death during induction therapy, OS, EFS and relapse.
For the selection of participants, inclusion criteria were being under the age of 18; having a diagnosis of de novo morphologic APL corresponding to subtype M3 or m3v with immunophenotyping by flow cytometry compatible with APL; presentation of t(15;17) translocation; having a diagnosis of atypical morphology APL but with t(15;17) translocation positive by RT-PCR; being on treatment based on the PETHEMA LPA 99 protocol since the induction phase in the hospital; having a normal echocardiogram result; and having the informed consent signed by the parents accepting the treatment.
Patients who did not have a report of t(15;17), due to unavailability of the test in the institution or to failures in test performance, were included in the protocol based on myelogram morphology and positivity of immunophenotyping by flow cytometry.
Patients diagnosed with AML other than APL, or who had received cancer treatment at another institution prior to admission to the Fundación Hospital Pediátrico La Misericordia were excluded.
Treatment
Participants received treatments based on the PETHE-MA LPA 99 protocol and according to their classification in the risk group, which included transfusion support management with platelets to maintain blood counts >30x109/L, cryoprecipitates to maintain fibrinogen above 100 mg/dL, and fresh frozen plasma to maintain clotting times less than twice the normal value. The protocol is described in Table 1.
Table 1 Institutional protocol for the treatment of acute promyelocytic leukemia based on the PETHEMA LPA protocol 99.
| Remission induction | ||
|---|---|---|
| Idarubicin | 12 mg/m2/day (days 2, 4, 6 and 8) | |
| ATRA | 25 mg/m2/day (from day 1 until complete remission) | |
| Prednisolone | 0.5 mg/kg/d (days 1-15) | |
| Consolidation (21-day cycles) | ||
| Low risk | Idarubicin | 5 mg/m2/day (days 1-4) + ATRA at a dose of 25 mg/m2/day (15 days) |
| Mitoxantrone | 10 mg/m2/day (days 1-5) + ATRA at a dose of 25 mg/m 2 /day (15 days) | |
| Idarubicin | 12 mg/m2/day (day 1) + ATRA at a dose of 25 mg/m2/day (15 days) | |
| Medium and high risk | Idarubicin | 7 mg/m2/day (days 1-4) + ATRA at a dose of 25 mg/m2/day (15 days) |
| Mitoxantrone | 10 mg/m2/day (days 1-5) + ATRA at a dose of 25 mg/m2/day (15 days) | |
| Idarubicin | 12 mg/m2/day (days 1 and 2) + ATRA at a dose of 25 mg/mm2/day (15 days) | |
| Maintenance (begins one month after consolidation and ends two years later) | ||
| ATRA | 25 mg/m2/day (15 days every 3 months for 6 cycles) | |
| Methotrexate | 15 mg/m2/day each week | |
| 6-mercaptopurine | 50 mg/m2/day | |
ATRA: All-trans retinoic acid.
Source: Own elaboration.
Risk classification
Low risk: patient with white blood cell count <10 000/L and platelets >40x109/L at diagnosis. Medium risk: patient with white blood cell count <10 000/L and platelets <40x109/L at diagnosis. High risk: patient with white blood cell count> 10 000/L at diagnosis.
Morphologic complete remission
This remission is defined as the absence of extramedullary leukemic infiltration accompanied by a hemogram with neutrophil counts >1.5x109/L and platelets counts>100x109/L, absence of atypical blasts or promyelocytes and a normocellular bone marrow aspirate with less than 5% blasts or atypical promyelocytes. The treatment protocol specifies a maximum time limit of 90 days from the start of ATRA treatment to achieve the aforementioned criteria.
Events
Death during induction: death occurring between the day induction treatment is started and before remission is documented at the end of induction treatment. Death during treatment: death occurring between the first day of treatment until the end of treatment. Relapse: having more than 20% of atypical blasts/promyelocytes in 1 bone marrow aspirate after entering remission, having more than 5% of atypical blasts/promyelocytes in 2 bone marrow examinations 1 week apart, or having the PML-RARa gene reappear in 2 consecutive bone marrow samples analyzed by RT-PCR at any time after the end of the consolidation phase.
Outcomes
Overall survival: time from diagnosis until death from any cause or until the last time the subject was known to be alive.
Event-free survival: time elapsed between initiation of treatment and early death, death during induction, disease relapse, development of second malignancy, or death due to toxicity; in patients who are alive and in remission, this moment will be the date of the last time the subject was known to be alive.
Statistical analysis
Qualitative variables were analyzed using frequencies and percentages, whereas quantitative variables were analyzed using averages, medians, and dispersion measures such as interquartile range, minimum value and maximum value. The Kaplan-Meier method and the log-rank test were used for survival analysis.
Ethical considerations
The ethical principles for medical research involving human beings established by the Declaration of Helsinki14 and the provisions on health research in Resolution 8430 of 1993 of the Ministry of Health of Colombia were followed.15 The present study is considered a risk-free research as no intervention was performed on the patients and only the information available in their medical records was analyzed. This research was endorsed by the Ethics Committee of the Fundación Hospital Pediátrico de La Misericordia through Minutes CEI 006-17 of May 17, 2017. In addition, the confidentiality of patients' personal data was ensured by re-identifying their medical records.
Results
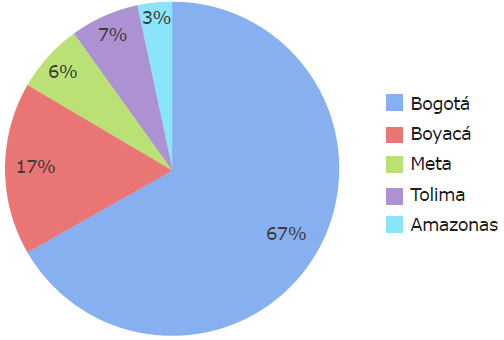
30 patients with a median age of 10 years were analyzed (range: 1-16 years); the majority were males (60%) and lived in Bogotá at the time of diagnosis (67%) (Figure 1).
Laboratory test findings at diagnosis in the patients analyzed are described in Table 2.
Table 2 Blood count findings at diagnosis.
| Test | Median | Range (max-min) | Interquartile range |
|---|---|---|---|
| Hemoglobin | 8.65 | 4.6-13.7 | 3.18 |
| Leukocytes | 6 045 | 1 200-350 000 | 24 617 |
| Platelets | 33 000 | 6 000-454 000 | 49 750 |
Source: Own elaboration.
The diagnosis of APL was made by morphology and flow cytometry in all cases, the immunophenotyping by flow cytometry being characteristic in 100% of the sample (Table 3).
Table 3 Phenotyping by flow cytometry at diagnosis
| Positive markers | n | % |
|---|---|---|
| Myeloperoxidase | 30 | 100 |
| CD 13 | 28 | 93 |
| CD 33 | 30 | 100 |
| CD 117 | 28 | 93 |
| CD 38 | 14 | 47 |
| CD 64 | 21 | 70 |
| CD 45 | 6 | 20 |
| CD 34 | 1 | 3 |
Source: Own elaboration.
Translocation, which was established by RT-PCR, was positive for t(15;17) in 18 patients (60%) and negative in 1 (3.33%); no data were obtained in 11 cases (37%) due to unavailability of the test in the institution or failure in the performance of the test. The patient with a negative result was treated with the PETHEMA LPA 99 protocol and was included in the analysis due to their cell morphology and typical clinical characteristics of APL.
The 5 (16.66%) patients with m3v variant were classified as high risk; of these, 3 died and 2 were alive at the time of the present research.
Relapses
Five patients relapsed, four with isolated involvement in bone marrow and one with both bone marrow and central nervous system involvement. Three of the patients who relapsed died and two were alive at the time of this study. Both survivors had isolated hematologic relapse with classic morphology, one was classified as high risk 1.5 years after diagnosis, and the other was medium-risk 3.5 years after diagnosis. These two patients achieved second remission with arsenic trioxide and received consolidation treatment with autologous hematopoietic progenitor cell transplantation.
Mortality
Of the 7 patients who died, 2 died within the first 24 hours of hospital admission, one from intracranial hemorrhage and one from leukostasis; neither was able to receive chemotherapy. Another patient was admitted with a massive intracranial hemorrhage and given supportive therapy; nevertheless, he died after 7 days and was unable to undergo chemotherapy due to his clinical condition. A fourth patient received chemotherapy according to the protocol but had severe epistaxis and intracranial hemorrhage and died after 12 days. Finally, 3 patients died after relapse: 2 relapsed during maintenance and died 10 and 12 months after diagnosis; the other achieved second molecular remission with the PETHEMA LPA 99 treatment protocol for relapse and consolidation with autologous hematopoietic precursor cell transplantation but died after a second relapse 3 months after transplantation and 3.5 years after diagnosis. Table 4 describes the characteristics of the patients who died.
Table 4 Description of patients in the cohort who died.
| Age and risk at admission - m3v morphology | Moment of death | Cause of death |
|---|---|---|
| 1. Male - 2 years (high risk) | Within the first 24 hours | Severe ICH |
| 2. Male - 15 years (high risk -microgranular) | Within the first 24 hours | Leukostasis (350 000 leukocytes at diagnosis) |
| 3. Male 5 years (high risk) | At day 7 | Massive ICH |
| 4. Male - 15 years (medium risk) | At day 12 | Severe ICH |
| 5. Female - 9 years (high risk -microgranular) | At 10 months | She relapsed and died during maintenance |
| 6. Male - 14 years (high risk -microgranular) | At 12 months | He relapsed and died during maintenance |
| 7. Female - 10 years (low risk) | At 42 months | Second relapse 3 months after -hematopoietic progenitor cell transplantation |
ICH: Intracranial hemorrhage.
Source: Own elaboration.
With regard to the social security scheme, 19 (63%) participants were from the contributory scheme and 11 (37%) from the subsidized scheme; no statistically significant differences were observed in OS between these two groups (Figure 2).
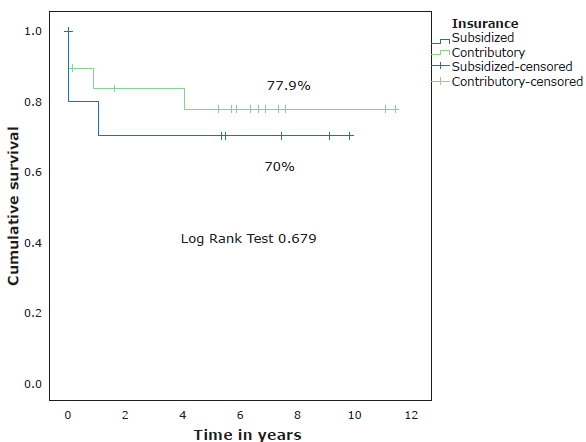
Source: Own elaboration.
Figure 2 Survival according to the social security scheme of the participants.
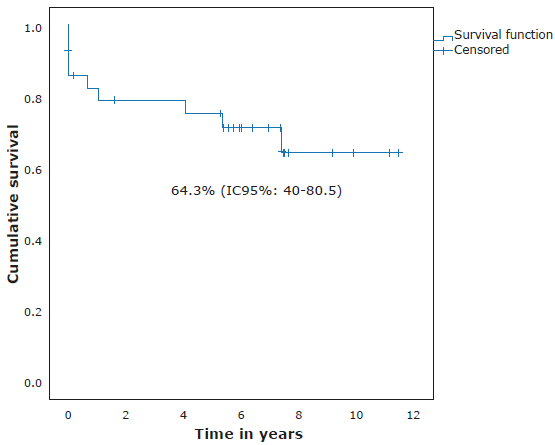
Concerning risk classification, 13 (43%) participants were classified as high risk, 12 (40%) as medium risk, and 5 (17%) as low risk. The 11-year OS, according to risk, was 59.2%, 91.7% and 80% for high-, medium- and low-risk patients, respectively (Figure 3). OS for all participants was 75.4% (95%CI: 55.1-87.5) (Figure 4), while EFS was 64.3% (95%CI: 40-80.5) with a median follow-up time of 6.35 years (0-11.43 years) (Figure 5).
Discussion
At present, there are few cohort studies on APL conducted exclusively in pediatric populations worldwide,16-18 especially in Latin America.19 20 Moreover, most of the results in this population are derived from research in adult patients. The present study compiles the experience on the treatment of APL at the Fundación Hospital Pediátrico La Misericordia for 7 years, with a follow-up time of up to 11.4 years.
These results provide insight into the response to treatment based on the PETHEMA LPA 99 protocol used for the management of this pediatric cohort. This protocol is based on the administration of ATRA combined with anthracycline chemotherapy, and its implementation achieved OS and EFS rates similar to those achieved in developed countries.6,9,11,18,21
Likewise, a predominance of the male sex in a ratio of 1.5:1 was found, which coincides with the findings reported in other articles.16,17,19 The proportion of patients classified as high risk (43%) was higher than that of the GIMEMA group (22.6%) but similar to that reported in Latin America (39.6%).9 EFS in the low-risk group was markedly affected because 1 of the patients relapsed and required management with the PETHEMA LPA 99 relapse protocol and, although his condition improved with an autologous transplant, he had a second relapse and died 3 months later, lowering the group's survival rate to 80%. In the medium-risk group, which had 12 patients, survival was 91.7%. These results are similar to those reported by Serephanoglu et al.9
Although early death is common in patients with APL, it occurred in only 10% of cases in this cohort; none of these patients reached the initiation of chemotherapy, which is consistent with most reports.9,16,18,19,22 The main cause of death was intracranial hemorrhage and the moment of greatest risk was the first 15 days after diagnosis, despite intensive transfusion support. Therefore, according to the PETHEMA LPA 99 protocol, it is essential to provide timely and adequate transfusion support to maintain safe hemostatic levels during the first two weeks after diagnosis; this is possible if the transfusion strategy of platelets, fibrinogen and other coagulation factors is implemented, and if support for the management of leukostasis is provided, aspects which were followed in the cohort analyzed here.6.9-13,16,19
The M3v variant of APL usually has a worse outcome; however, the sample analyzed in the present study was too small for statistical analysis.23,24.
Finally, immunophenotyping by flow cytometry in the studied population was positive for the MPO, CD 13, CD 33, CD 117 and CD 64 markers, similar to the findings described by Orfao et al.25
One of the limitations of the present study was its retrospective nature and the fact that it was not possible to perform a molecular analysis on all patients. In addition, a limited number of participants were analyzed. However, it should be noted that these patients were exclusively children under 18 years of age at diagnosis and were treated at only one health institution and that APL should be considered a rare subtype of leukemia.
Lastly, and to consider in further studies, it is necessary to perform an individual analysis of the immunophenotype marker CD 56 since some publications mention it as a poor prognostic factor.26,27
Conclusion
Although OS values were lower than those described in pediatric populations in other countries, the implementation of the PETH EMA LPA 99 protocol i n the treatment of APL in the study population, in general, showed satisfactory results. Therefore, its use in the pediatric population is suggested, taking into account the adjustments recommended by the protocol regarding the characteristics of this age group.