Introduction
Antibiotic resistance is a public health problem that has increased worldwide in recent years. In Europe, for example, the European Centre for Disease Prevention and Control, cited by Carlet et al.,1 reported that about 25 000 people die each year from infections caused by antibiotic-resistant bacteria.
Carbapenems are a subclass of antibiotics with the broadest spectrum; they have high efficacy and safety profile compared to other therapeutic alternatives against Gram-positive and Gram-negative bacteria and are considered a fundamental resource for the treatment of infections by resistant microorganisms. Thus, the emergence and spread of antibiotic resistance is a major public health concern.2
There are microorganisms such as Stenotrophomonas maltophilia3 that are intrinsically resistant to carbapenems; however, most bacteria acquire resistance to these antibiotics. For example, in the 1990s, chromosomal metallo-β-lactamases (MBL) were discovered in some carbapenem-resistant isolates of Pseudomonas aeruginosa, later were detected in species of the genus Acinetobacter, and most recently were shown to have migrated to Enterobacteriaceae.4
Gram-negative bacteria have developed multiple resistance mechanisms: some species prevent carbapenems from reaching penicillin-binding proteins by reducing permeability in the cell membrane, while others actively expel canapenemases with efflux pumps.5 The production of β -lactamases is the most important form of resistance at the clinical and epidemiological level; thus, the carbapenems most recognized for their hydrolytic capacity and international dissemination power through high-risk clones are Klebsiella pneumoniae carbapenemase (KPC), Verona Integron-encoded Metallo-β-lactamase (VIM), New Delhi MBL (NDM), imipenemase (IMP), and oxacillinase (OXA)-48-like carbapenemase. In Latin America, the first carbapenemase-producing bacteria to be identified was KPC and the first country to do so was Colombia in an isolation of K. pneumoniae.6,7
In the order of Enterobacterales, the Enterobacteriaceae family provides the largest number of species that can be resistant to carbapenems, which can cause serious infections such as bacteremia, pneumonia, and complicated urinary and intra-abdominal infection.8 The most frequently identified form of resistance in these organisms is the generation of enzymes capable of hydrolyzing β -lactam antibiotics such as carbapenems; these bacteria are known as carbapenemase-producing Enterobacterales (CPE).9
In 2017, the World Health Organization (WHO)10 released a priority list of antibiotic-resistant bacteria that included 12 families of pathogens in order to guide and promote research on the subject and develop new antibiotics. In this group, CPEs were considered a critical priority because the infections they cause can increase complications and mortality, the latter estimated at about 40% by Ramos-Castañeda et al.11 Similarly, in a systematic literature review, Martin et al.12 reported a significantly higher risk of overall mortality (OR: 3.39, 95%CI: 2.35-4.89) compared to infections caused by microorganisms sensitive to carbapenems, which coincides with the studies conducted in Colombia by Gualtero et al. 13 and Cienfuegos-Gallet et al.14
The health costs generated by infections caused by CPE are higher than those caused by other infections, such as influenza, or chronic conditions such as asthma, high blood pressure, or diabetes. In this regard, Bartsch et al.15 state that costs increase proportionally with the incidence of infection, increasing 2.0, 3.4 and 5.1 times for incidence rates of 6, 10 and 15 per 100 000 persons, respectively. In Colombia, where KPC-producing bacteria are endemic, Vargas-Alzate et al.16) describe an increased health care cost in patients with infections caused by carbapenem-resistant enterobacteria; for example, in the case of urinary tract infection, costs are USD 633 higher than in individuals whose infections are caused by microorganisms sensitive to β -lactams.
The aim of the present research was to develop a guideline that makes recommendations based on scientific evidence and adapted to the Colombian context. This is a joint effort of the Hospital Universitario Nacional de Colombia, Universidad Nacional de Colombia, the District Health Department of Bogotá, Pontificia Universidad Javeriana, Asociación Colombiana de Infectología and other institutions in Bogotá.
The recommendations made here are intended to be implemented in Colombian healthcare centers by infection control groups (infection committees, infection prevention committees, healthcare-associated infection committees or those in charge of these activities), as well as by general practitioners or specialists involved in the clinical care of patients with these types of infections, including internists, critical care specialists, infectiologists, etc. They can also be implemented by nurses, pharmaceutical chemists, microbiology professionals (bacteriology, microbiology) and administrative staff involved in the treatment of infections caused by CPE.
Objectives
Given this scenario, the aim of this study was to develop a clinical practice guideline (CPG) for the screening of patients at risk of colonization by CPE and the treatment of patients with suspected or confirmed infections caused by this type of bacteria through a process of adaptation of CPGs based on the ADAPTE methodology.17 Similarly, derived from this objective, it was intended to make useful recommendations for screening and timely identification of CPE carriers admitted to hospitals in order to initiate appropriate antibiotic treatment, taking into account the clinical scenario and the factors that increase resistance.
Aspects addressed by the guideline
The proposed CPG comprises two main topics: the screening of patients at risk of colonization by CPE and the pharmacological treatment of individuals with suspected or confirmed infection by these microorganisms.
Target patients
The guidelines were designed to be implemented in adult patients (over 18 years of age), treated or hospitalized in healthcare centers, who are at risk of colonization with CPE, and in whom there is clinical suspicion or confirmation of infection by these microorganisms.
Users
The recommendations set forth in this CPG are intended for health care teams performing infection control activities or caring for adult patients at risk of colonization with CPE, or with suspected or confirmed infection by such microorganisms. These teams include healthcare professionals such as general practitioners or specialists in emergency medicine, internal medicine, intensive care, infectious diseases and infection control or prevention, as well as professionals working in clinical laboratories (bacteriology or microbiology) and nurses.
The guidelines are also aimed at health care decision-makers, both collective and individual, working in clinical, administrative, or financial areas in hospitals and health insurance companies.
Methodology
The ADAPTE methodology was used for the development of this CPG,17 as it allows adapting or modifying recommendations already established for a specific scenario so that they can be used in different settings. This methodology is a rational option for generating new CPGs.
The purpose of using the ADAPTE methodology is to develop and implement new CPGs from existing CPGs more effectively in order to acquire a high level of quality and ensure recommendations that take into account particular and relevant health aspects in the new context in which they will be utilized. It also intends to abide by specific requirements, legal conditions, regulations, priorities, and political and budgetary availability of the institutions.
The recommendations contained in this CPG were established using participatory methods based on a systematic search and identification of scientific literature, as well as on an assessment of the epidemiological context and the operation of the Colombian health system. This process was carried out in the following order:
Step 1
The topics to be covered in the consensus document were defined, and specific questions to be resolved were raised in accordance with the need to properly identify and treat CPEs. The topics and questions were selected based on the experience of the guideline development group (GDG).
This first step allowed establishing the global terms of reference, the feasibility of the adaptation in terms of information availability, the methodology to be employed, the needs identified, and the process planning.
The GDG consisted of an infectologist (JAC), a microbiologist with training in infection control (ALL), and three internal medicine specialists (GAM, JSB, lCnB). It should be noted that the infectiologist (JAC) has experience in the development of CPGs.
Step 2
Questions were formulated following the PICO format and systematic literature searches were made in databases (PubMed and Embase) and GPC sites (SIGN, Guideline Central, etc.) to answer each of them.
Moreover, during this second stage, the criteria for inclusion of the documents to be selected were defined; this process is described in Annex 1. The GDG was responsible for the initial searches and the selection process.
Step 3
Once the systematic search of the literature was carried out, the documents and studies that contributed to the resolution of the questions posed in step 2 were selected, and their methodological quality was evaluated using the AGREE II instrument.18 This instrument evaluates several aspects related to methodology, quality, clarity, and relationship with the sponsors of the CPG, as well as its validity.
For the evaluation of the CPGs, the time of publication and the periods in which the scientific literature was searched for were considered in order to obtain the most recent evidence. The content of the CPGs was assessed by identifying whether the recommendations were adequately supported by evidence and whether there was consistency with the respective graded levels. Likewise, consistency was evaluated by analyzing how articles supporting the recommendations were searched for and selected and by establishing whether there was a correlation between the evidence reported in the literature and how it was summarized and interpreted, and between how information was interpreted and recommendations were formulated.
Finally, it was determined whether the recommendations were acceptable and valid in the Colombian context and according to the operation of the national health system and the financial resources available in the country.
Step 4
Finally, the CPGs identified by the GDG members were evaluated, all recommendations were collected, and their potential for implementation was defined. Four documents were selected for this process: "Guidelines for the prevention and control of carbapenem-resistant Enterobacteriaceae, Acinetobacter baumannii and Pseudomonasaeruginosa in health care facilities",19 "Screening for carriage of carbapenem-resistant Enterobacteriaceae in settings of high endemicity: a position paper from an Italian working group on CRE infections",20 "Israeli National Policy for Carbapenem-Resistant Enterobacteriaceae Screening, Carrier Isolation and Discontinuation of Isolation",21 and "French recommendations for the prevention of 'emerging extensively drug-resistant bacteria' (eXDR) cross-transmission".22
The recommendations were made based on the data gathered during the evaluation process described above, which was carried out using the GRADE approach23 and with the participation of experts. Likewise, in this step, concordance between the established recommendations and the articles on which they were based was evaluated.
The GRADE approach has the following elements of judgment to define the strength and direction of recommendations: a) problem prioritization; b) benefit of desirable outcomes; c) desirable and undesirable effects; d) certainty of the evidence of adverse outcomes; e) uncertainty concerning values and preferences of patients, or their variability among patient, about each outcome; f) balance between gains and the risks and drawbacks of the recommendations; g) costs and resource use; h) certainty of economic requirements; i) cost-effectiveness of recommendations; and j) equity of recommendations.
Recommendations were categorized according to the level of evidence and each category pointed out two aspects: 1) the level of reliability given to the estimated effect (beneficial or adverse) of the intervention, so that the recommendations are for or against it, and 2) the level of certainty available to define whether its favorable effects outweigh the adverse effects (Annex 1).
Health professionals who participated in the development of this CPG declared whether they had conflicts of interest regarding the development of the guideline in general or the recommendations in particular; this form included information about several areas that may or may not be related to the aspects defined in the CpG and was completed before starting the preparation of the document and holding the consensus meeting.
Questions
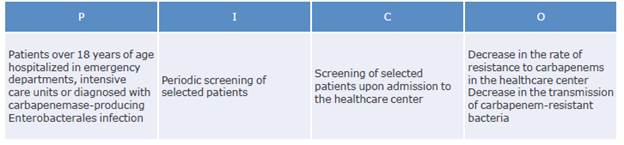
Which patients should be screened for colonization with carbapenemase-producing Enterobacteriaceae?
What is the recommended technology for screening hospitalized patients colonized with carbapenemase-producing Enterobacteriaceae?
How often should screening tests for carbapenemase-producing Enterobacteriaceae be performed in selected patients?
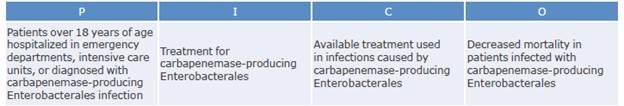
Which antimicrobials can be used to treat infections caused by carbapenemase-producing Enterobacteriaceae and what is the best management strategy?
It should be pointed out that the development of this CPG involved the participation of health professionals from different areas who were able to guide the recommendations and assess potential organizational barriers or barriers to the implementation of the CPG. An update of these recommendations is expected to be completed within 5 years.
Recommendations and evidence review
Question 1: Which patients should be screened for colonization with carbapenemase-producing Enterobacterales?
Recommendations
Active surveillance for colonization with CPE is recommended. Strength of recommendation: strong for. Quality of evidence: low (⊕⊕ΟΟ).
We recommend screening for asymptomatic colonization based on local epidemiology and risk assessment. Strength of recommendation: strong for. Quality of evidence: low (⊕⊕ΟΟ).
We recommend screening for CPE infection in patients with previous CPE colonization; who have been in contact with other patients colonized or infected with CPE;
who have a history of hospital stay >24 hours in the last 6 months; who have been admitted to intensive care units (ICU); patients in dialysis, chemotherapy, chronic care, oncology, transplant or hemato-oncology units; patients readmitted to ICUs; patients who have been treated with carbapenems; and patients referred from any other healthcare center. Strength of recommendation: strong for. Quality of evidence: low ((⊕⊕ΟΟ).
Recommendation rationale
Due to the clinical significance (morbidity and mortality) of CPE infections, it is considered necessary to perform active and continuous surveillance in patients who have been or may be infected with this type of bacteria.
Most of the studies that evaluated the usefulness of screening for colonization with CPE showed that the number of infections caused by these microorganisms decreases when screening tests are performed in at-risk groups through weekly cultures; these results were associated with other interventions such as contact isolation measures, education of healthcare personnel, improved adherence to hand hygiene, and optimization of antimicrobial formulation.
The consensus panel, as a whole, considered that the strength of these three recommendations was strong for the intervention, despite having a low-quality evidence, because they take into account that Enterobacteriaceae resistance to carbapenems in Colombia is a public health problem with high rates of sustained resistance over time and that there is an endemic circulation of CPE in the country.
Risk factors for colonization with CPE include previous infection by this microorganism, prolonged hospital stay (especially in critical care, dialysis, transplantation units, etc.), and previous use of carbapenems.
Early identification of patients infected with CPE allows timely isolation; nevertheless, it should be noted that these patients do not require treatment.
Evidence
The prevalence of resistance to carbapenems in clinical isolates in Colombia varies considerably depending on the type of microorganism, the population studied and the geographic area where the infection is contracted, with results as low as 1% for Escherichia coli, or as high as 23% for K. pneumoniae and 60% for Providencia rettgeri.24,25
Furthermore, in Colombia, it has been established that in 89% of CPE isolates, resistance is mediated by the production of carbapenemases, while the remaining 11% is explained by other mechanisms such as hyperproduction of AmpC S-lactamases and porin mutations.24-26
It has been established that the most common carbapenemases worldwide are KPC (65%) and NDM (22%).27,28 Although data are presented in a general way, this information, particularly the local epidemiology, is critical for healthcare centers because evidence suggests that most of the circulating CPE in Colombia produce Class A and B carbapenemases. Therefore, infection control programs should focus their efforts on detecting these antimicrobials, which, besides being the most common, have the greatest impact on public health and the dissemination of resistance in hospitals.27-29 In this sense, local information is decisive to define the applicability of the recommendations provided here, as well as the best strategies for identifying CPEs and the most effective treatment.
The studies found in the systematic literature search that support the second recommendation and active surveillance in patients with asymptomatic CPE colonization have assessed the impact of screening along with other strategies applied simultaneously, highlighting the importance of always combining screening with other interventions to reduce the dissemination of resistance.
The reviewed literature presented multiple interventions used on patients with CPE infections, which can be combined in a variety of ways. For example, Viale et al.30 conducted a quasi-experimental study in a university hospital in Italy in which they compared a pre-intervention period (June 2019 to July 2011) with an intervention period (August 2011 to January 2014) to assess the impact of an infection control program on the incidence of CPE. They found a significant decrease in the incidence rates of bloodstream infections caused by these microorganisms (risk reduction: 0. 96, 95%CI: 0.92-0.99, p=0.03) and of colonization with CPE (risk reduction: 0.96, 95%CI: 0.95-0.97, p<0.0001) in the second study period, thus proving the benefit of multidisciplinary intervention based on asymptomatic screening.30
Hayden et al.31 conducted a study in which they found that combined intervention was significantly associated with a reduction in cases of KPC colonization and infection in four Chicago long-term acute care hospitals with a high endemic prevalence of KPC (p=0.004).
In turn, Gagliotti et al.32 published a study with the results of the implementation of a series of measures to prevent CPE infections in the Emilia-Romagna region of Italy. They included confirmation of CPE infection by phenotypic testing; active surveillance of asymptomatic CPE carriers through rectal swabs for close contacts of hospitalized patients with CPE, patients transferred from other hospitals or from endemic countries, and patients admitted to ICU or transplant, oncology and hematology units; isolation for patients infected with CPE and asymptomatic carriers during their hospital stay; and reporting of the presence of CPE at the time of patient transfer. The authors found that the intervention was effective in reducing the incidence rate of CPE infections from 32 cases per 100 000 hospital patient days to 15 cases per 100 000 hospital patient days.
In a larger study conducted in a hospital of Israel, Ciobotaro et al.33 evaluated the implementation of a multidisciplinary strategy over a 3-year period that included active surveillance of patients at high risk for CPE colonization; guidelines for patient isolation, cohorting, and environment cleaning; and education of staff. Active surveillance was performed by means of rectal swab cultures taken only once from asymptomatic patients in contact with individuals with CPE infection or colonization, admitted to the ICU or transferred from another hospital. The authors found that the incidence of KPC infections had a 16-fold decrease, while the cross-infection rate went from 6% to 2.7% after this intervention.
The studies presented above show the benefit of active surveillance by rectal swabbing, as long as it is implemented in along with contact isolation measures and with a third or fourth factor that may include the training of healthcare personnel, optimization of hand hygiene, or a decrease in the formulation of carbapenems through antimicrobial use optimization programs.
The risk groups chosen for active surveillance differed somewhat among papers, which could be attributed to the fact that the populations studied were drawn from both endemic and non-endemic regions for CPE and therefore the protocols were heterogeneous. Regardless of these differences, what should be emphasized about screening in these studies is that none of them systematically tested all patients admitted to the centers and that, in all hospitals, the population for active surveillance was specifically selected. These strategies should be adopted in Colombia bearing in mind the epidemiology of each healthcare center and using the patient groups proposed in the studies described above as a guide.
Based on these studies, it is also possible to establish previous colonization, prolonged hospitalization, prolonged stay in long-term care facilities, and the use of invasive devices (orotracheal tubes, endovenous catheters, bladder catheters, etc.), mechanical ventilation and antibiotics (especially carbapenems and quinolones) as risk factors for CPE colonization and infection.34-38
Question 2: What is the recommended technology for screening hospitalized patients colonized with carbapenemase-producing Enterobacterales?
Recommendation
1. We suggest that each center defines the technology to be employed based on the algorithm described below (Figure 1 and Table 1), considering the prevalence of CPE and the availability of resources. Strength of recommendation: weak for. Quality of evidence: very low ⊕ΟΟΟ.
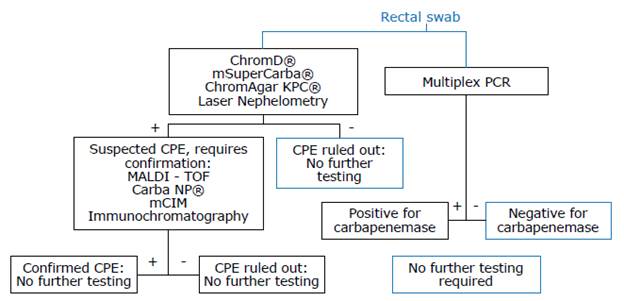
Source: Own elaboration.
Figure 1 Algorithm for screening and confirming infection with carbapenemase-producing Enterobacterales.
Table 1 Diagnostic performance of tests available in Colombia for screening and confirming infection with carbapenemase-producing Enterobacterales.
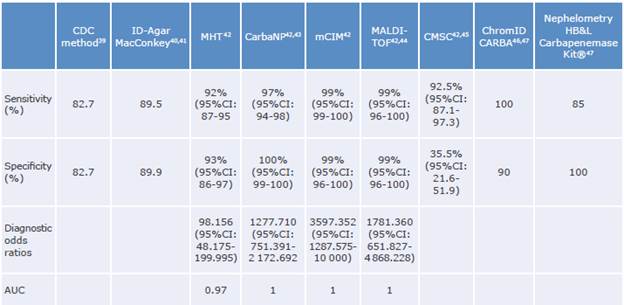
CDC: Center for Desease Control and Prevention; MHT: modified Hodge test; CarbaNP: carbapenemase Nordmann-Poirel test; mCIM: modified carbapenem inactivation method; MALDI-TOF: matrix-assisted laser desorption ionization-time of flight mass spectrometry; CMSC: CHROMagar™ mSuperCARBA™; CI: confidence interval; AUC: area under the curve.
Source: Own elaboration.
Good clinical practice points
We suggest estimating CPE infection every 2 months if the prevalence of CPE infection in the healthcare center is <15%.
Laser nephelometry as a screening test when the prevalence of CPE infection is ≥15% in the healthcare center is not recommended. In addition, we suggest repeating this test with a new rectal swab sample if the initial screening was negative, because its low sensitivity and low negative predictive value are not sufficient to rule out CPE infection in high-prevalence settings.
We do not recommend performing a confirmation test using CarbaNP if the prevalence of CPE infection in a healthcare center is ≥15%, since its negative predictive value is insufficient to rule out an infection of this type with certainty. Therefore, repeating the confirmatory test with another method is suggested.
The screening test performed on MacConkey agar with meropenem disks, or supplemented with meropenem, could be used in low or high prevalence scenarios if the clinical laboratory previously performs standardization and validation tests to improve the reliability of results. This test may be an option in care centers with limited resources, where the purchase of chromogenic agars or laser nephelometry equipment is limited.
Recommendation rationale
Screening for colonization with CPE may be performed on samples obtained by rectal swabbing.
Nasal swabs, pharyngeal swabs, bronchial aspirates, and urine cultures in patients with bladder catheters are alternatives for sampling that should be considered when there is direct suspicion of infection or colonization with CPE in these sites.
There are several methods available for screening in-patients for CPE colonization; however, there is no evidence demonstrating the superiority of any of them in terms of reducing the frequency of nosocomial infections or transmission of CPE.
We recommend performing microbiological tests to screen for CPE colonization if confirmatory tests for carbapenemase production are also performed or, alternatively, tests that allow screening for CPE colonization directly from the rectal swab without requiring initial microbiological isolation (molecular tests).
Since there is no evidence to establish that one test is superior to the others, each healthcare center should define the test to be used based on its CPE infection prevalence and resource availability. The proposed algorithm (Figure 1, Table 1) summarizes the most relevant data regarding diagnostic performance that should be considered when making such decisions.39-47
The proposed algorithm (Figure 1) enables the user to select between a screening test and a confirmatory test or a polymerase chain reaction (PCR) test. Importantly, due to the high sensitivity and specificity of PCR tests, PCR test results do not require confirmation.
The tests (or set of tests) should be selected according to the availability of resources at each healthcare center.
Evidence
Diagnostic tests for screening patients at risk of CPE colonization are scarce. However, there are technological alternatives, such as microbiological culture tests, laser nephelometry and tests based on molecular methods, which should be performed depending on their availability in each healthcare center.48 These tests can be classified as molecular or phenotypic:
Molecular tests: They identify resistance genes and have the advantage of detecting and differentiating enzymes directly, thus facilitating the screening process. They include the PCR test, which can be performed on agar colonies or directly on rectal swab or stool samples; it has a high sensitivity and specificity and is considered confirmatory. Another advantage of the PCR test is that direct detection in rectal swabs saves time, allowing rapid definition of the need for further isolation and correct identification of resistance mechanisms.49 There are several techniques in Colombia that allow for the use of this approach, but its primary constraint is financial, and unfortunately, there are no cost-effectiveness studies that allow for protocolization of its proper application.
Phenotypic tests: They identify (or suggest) resistance and can be classified depending on their ability to capture or screen for potential resistance; their turnaround times are relatively short, and they can identify the type of enzyme produced in differentiation and classification tests. Some of these tests are described below.
In Atlanta, the Centers for Disease Control and Prevention39 developed a screening test in which the rectal swab sample is emulsified in 5 mL of Trypticase soy broth (TSB) and then supplemented with a 10-μg carbapenem (ertapenem or meropenem) disc. This mixture is incubated overnight and subsequently subcultured onto MacConkey agar to be incubated for an additional night. If no bacterial growth is observed after 48 hours, it is considered negative; nevertheless, if growth is observed, the species must be identified and confirmation tests for carbapenemases must be performed, requiring up to 4 days for a final result. Both the sensitivity and specificity of this approach are 82.7%.
Direct inoculation on a MacConkey agar plate containing a carbapenemic sensidisc is a simple and cost-effective method for detecting suspected CPE colonies in good-quality samples.40,41 Similarly, it has been suggested that using a meropenem disc with boronic acid allows the isolation of KPC-producing bacteria; however, these discs do not allow for the differentiation of enzymes such as OXA-48, VIM and NDM. The result of direct inoculation is obtained in 16 to 24 hours.40
The modified Hodge test has a high sensitivity for finding KPC and OXA-48, but low sensitivity for detecting MBL. In addition, it can frequently yield false positive results with cephalosporinases such as extended-spectrum and AmpC β-lactamases (ESBL). This test provides results in 18 to 24 hours, is less expensive than direct inoculation of rectal swabs in specific selective chromogenic agars containing carbapenems (although the latter allows for direct study of certain carbapenemases), and its diagnostic performance is variable, with a sensitivity of 80-90% and a specificity of 60-90%.49
Other phenotypic tests are described below:
CHROMagar™ mSuperCARBA™ (France): It provides results within 24 hours and detects OXA-48, KPC, NDM, VIM and IMI.45 One of its advantages is that rectal swabs, perineal swabs, stool, and even urine can be used as samples.
ChromID® CARBA (France): This agar also allows direct detection of OXA-48, KPC and NDM-1; rectal swab and fecal samples can be inoculated on it. The estimated time to obtain a result is 18 to 24 hours and it has a good yield.46
Carbapenem inactivation method: It is based on the hydrolysis of a 10μg meropenem sensidisc incubated with a bacterial suspension in Trypticase soy broth. Results are obtained within 18 to 24 hours, and although the addition of ethylenediaminetetraacetic acid facilitates MBL differentiation, this test does not detect enzyme co-productions.40,46Laser nephelometry: It is a technique in which the intensity of scattered radiation is measured as it passes through a suspension of colloidal particles. The vials contain a suspension of carbapenems and are inoculated with the rectal swab sample; CPE is detected in approximately 6 hours. In Colombia, it was established that this methodology has a good performance in detecting CPE (sensitivity of 85% and specificity of 100%).47
Carba NP: It is an acidimetric confirmatory test that detects KPC, NDM-1, VIM, IMP, and OXA-48 producing bacteria from the pH change generated during imipenem hydrolysis following contact with a bacterial lysate.42 The time required to obtain the results is 30 minutes to 2 hours, but the total time must include the time required for the first culture, which is typically 24 hours.43
Matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF): It is based on the separation of particles according to their mass; in order to detect carbapenemases specifically, the microorganism is incubated with carbapenems, and the protein peaks formed during hydrolysis are recognized. The time to obtain the result is 4-6 hours and its yield depends on the expression and type of enzyme.44Semi-automated microbiology systems: They allow, besides identifying the species, to establish carbap-enem-resistant microorganisms and to infer the presence of CPE through expert software. However, they require additional confirmatory testing due to the limited sensitivity established to date.50 Some of the systems available in Colombia are Phoenix™, MicroScan™, and Vitek-2™.
In summary, the algorithms for screening patients at risk of colonization by CPE recommended in this CPG offer several alternatives with specific yields that overall allow >90% certainty of the presence of a carbapenemase. However, as previously stated, due to the lack of greater certainty regarding the diagnostic performance of the various tests and the lack of a cost-effectiveness analysis of these tests in the country, each healthcare center must analyze their options for establishing a diagnostic pathway to identify suspected cases and confirm the presence of a resistance mechanism.
Likewise, it is critical to keep in mind that, due to the costs of the tests, the use of certain technologies may result in increased inequity; in other words, hospitals with limited economic capacity would be at a disadvantage compared to hospitals with the possibility of obtaining certain technologies and controlling better the spread of these multidrug-resistant microorganisms.
Question 3: How often should screening tests for carbapenemase-producing Enterobacterales be performed in selected patients?
Recommendations
Rectal swab screening once a week until hospital discharge or until colonization with CPE is demonstrated in patients in services at high risk of infection is suggested. Strength of recommendation: weak for. Quality of evidence: very low ⊕ΟΟΟ.
We suggest using a single rectal swab sample for screening patients who meet the criteria for screening on admission to the healthcare center but do not require hospitalization in high-risk services. Strength of recommendation: weak for. Quality of evidence: very low ⊕ΟΟΟ.
Recommendation rationale
a. Although evidence on the optimal frequency of screening tests is scarce, of very low quality and heterogeneous, studies frequently design a program with weekly or biweekly screenings.
Evidence
Ambretti et al. ,20 Solter et al.21 and Lepelletir et al.22 recommend screening for CPE every week, while the WHO19 recommends screening every one to two weeks; however, there are no primary studies regarding the optimal frequency of screening. In this sense, the CPG proposed here seeks to ensure that patients at a higher risk of colonization with CPE during hospital stay can be identified in a timely manner.
Question 4. Which antimicrobials can be used to treat infections caused by carbapenemase-producing Enterobacterales and what is the best management strategy?
Recommendations
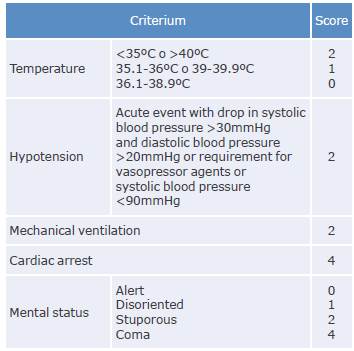
1. Estimating the mortality score using the INCREMENT-CPE instrument (Tables 2 and 3) in patients with CPE bacteremia and to initiate treatment with combination therapy in those with values ≥8 is recommended.
Strength of recommendation: strong for. Quality of evidence: low ⊕⊕ΟΟ.
2. Combination therapy (ceftazidime/avibactam in combination with carbapenems, polymyxins, tigecycline, aminoglycoside, fosfomycin sodium or fluoroquinolones) as the first line of treatment for Class A KPC infections is suggested. Strength of recommendation: weak for. Quality of evidence: low ⊕⊕ΟΟ.
3. We suggest starting treatment with polymyxin B or colistin in combination with carbapenems, tigecycline, aminoglycoside, fosfomycin sodium or fluoroquinolones when ceftazidime/avibactam is not available or when patients present resistance to the latter. Strength of recommendation: weak for. Quality of evidence: low ⊕⊕ΟΟ.
Table 2 INCREMENT-CPE risk score.
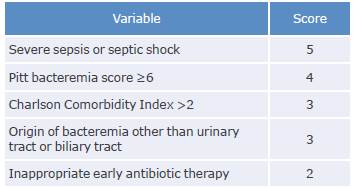
Note: The cut-off point for defining high mortality risk and need for combination therapy is established when the score is ≥8.
Source: Elaboration based on Gutiérrez-Gutiérrez et al.51
Good practice points
Antibiotic therapy should be initiated considering the anatomical site of infection, the mortality risk score, the local epidemiology, and the availability of antibiotics. In addition, this therapy can be adjusted based on the patient's clinical diagnosis, type of isolate, susceptibility profile, minimum inhibitory concentration (MIC), and adverse events and drug contraindications. Depending on each case, it is also possible to choose between monotherapy or combination therapy.
It is recommended to use combined therapy in the case of CPE infections, except for bacteremia secondary to urinary tract infection in the absence of MBL, and to consider the use of monotherapy in low-risk patients.
If the patient has intermediate sensitivity to the second antibiotic of the combination therapy scheme or if a second drug with in vitro susceptibility is not available, a third antibiotic should be added.
The use of meropenem should be considered in combination therapy when the MIC of the isolate against this antibiotic is ≤8 μg/mL.
If possible, the mechanism of carbapenem resistance should be confirmed, including the presence of MBL, OXA-48 enzymes, or enzyme co-productions.
If the presence of MBL (NDM, VIM, etc.) or enzyme co-production (KPC + VIM) is confirmed, adding aztreonam should be considered.
Recommendation rationale
There are no randomized clinical studies on the management of patients with infections associated with CPE, but the research retrieved from the literature review suggests a benefit of using combination therapy, especially in patients with more severe disease. It should be noted that the drugs available for the treatment of these infections may have low efficacy when used in monotherapy, which is especially true for polymyxins; in the latter case, the application and interpretation of susceptibility testing is controversial, and automated systems may have low sensitivity (<70%).
The ceftazidime/avibactam combination has been proven to have adequate in vitro susceptibility in a large amount of CPE isolates. However, its clinical benefit is not well documented, as there are only case series and a few cohort studies in which there seems to be no difference in efficacy when used alone or in combination.
Beta-lactams in general, and the ceftazidime/avibactam combination in particular, have a better safety profile than polymyxins, which is why their use is preferred as the basis for combination therapy schemes.
Cohort studies have shown that urinary and biliary tract infections due to CPE have a lower mortality rate than infections located in other organs and, therefore, they could be treated with a single drug.
Evidence
The efficacy of active drugs studied in vitro in monotherapy for the treatment of CPE infections has not yet been clearly determined; moreover, it is considered that certain combinations with antimicrobials may generate synergistic or additive effects. In this regard, Rodríguez-Baño et al.53 state that in vitro and in vivo studies have evaluated the effects of using double and triple therapies with drugs that have different mechanisms of action. However, there are no randomized clinical trials comparing combination therapy with monotherapy for CPE infections, in part because their design, conduct, and interpretation are complex due to the heterogeneity of the populations treated and the drugs and doses used, making it extremely difficult to synthesize the evidence.
So far, there are only observational studies comparing outcomes of patients with CPE infection treated with monotherapy or combination therapy. However, it is important to treat the patient not only from a pharmacological perspective but also keeping in view the focus of the infection, as controlling it may be critical for reducing the risk of mortality.13
Falagas et al.,54 in a systematic review of 20 studies that included 692 patients who received definitive treatment, compared mortality in patients with CPE infection treated with combination therapy and monotherapy, finding rates of 60% and 80%, respectively.
Zusman et al.55 performed a systematic review and meta-analysis in which they assessed the evidence of in vitro synergy of polymyxin-carbapenem combination therapy against Gram-negative bacteria and found that mortality is lower with this type of therapy than with monotherapy. This same study exposed the biases identified in the studies on combined therapy, reporting that when it is evaluated mainly as targeted therapy, there is survival bias and confounding by indication bias because the probability of receiving combined therapy is greater for the most critically ill patients; also, the definition of exposure to different treatments is heterogeneous, and there are inconsistent criteria for the number of days of treatment.
On the other hand, van Duin56 established that there are studies on CPE performed in populations that are not significant and in which the control of confounding factors was not sufficient.
Finally, Gutiérrez-Gutiérrez et al.51 conducted a retrospective study on 480 patients with bacteremia caused by CPE enrolled in the INCREMENT cohort who were treated in 26 tertiary care hospitals across 10 countries. They compared 30-day all-cause mortality in patients who received appropriate or ineffective therapy using the INCREMENT-CPE scale; among patients who received adequate therapy, they made comparisons between those who received monotherapy and those who received combination therapy using a preference to receive combination therapy score and a validated mortality score. The researchers found that appropriate therapy was associated with lower mortality than inappropriate therapy (38.5% vs. 60.6%). They also established that overall mortality was not different between those who received combined therapy or monotherapy, although the former was associated with lower mortality than the latter in patients with high mortality scores (48% vs. 62%). A subsequent validation showed similar performance of the INCREMENT-CPE scale.57
Discussion
The following is a discussion of the available evidence on the therapeutic alternatives used in the treatment of infections caused by CPE (Tables 4 and 5).
Table 4 Antibiotics used for the treatment of infections caused by carbapenemase-producing Enterobacterales.
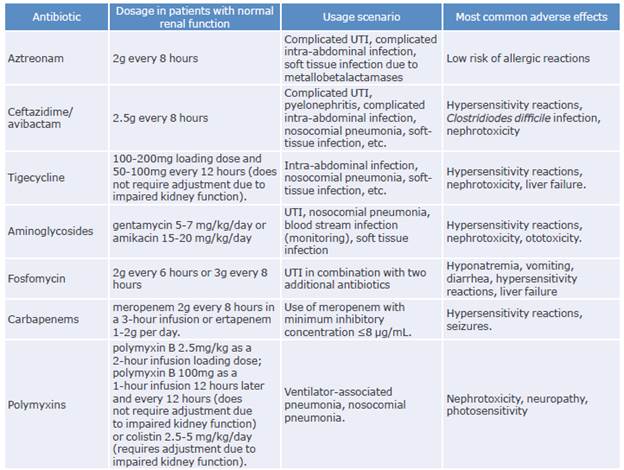
UTI: urinary tract infection.
Source: Elaboration based on Cunha & Cunha.58
Table 5 Adjustment of drugs used in the treatment of carbapenemase-producing Enterobacterales infections based on renal function.
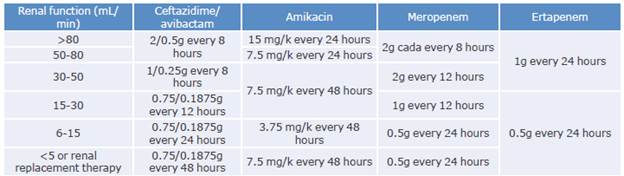
Source: Elaboration based on Cunha & Cunha.58
Ceftazidime/Avibactam
It is a combination of a third-generation cephalosporin and a p-lactamase inhibitor approved by the Food and Drug Administration (FDA) and the European Medicines Agency for the management of complicated urinary tract infection (including pyelonephritis), complicated intra-abdominal infections (metronidazole is added in this context), and hospital and ventilator-associated pneumonia. Similarly, in Europe, it is used to treat infections caused by Gram-negative bacteria when no other therapeutic options are available.
Avibactam has in vitro activity and inhibits class A (ESBL and KPC), class C (chromosomal and plasmid AmpC p-lactamases) and class D (OXA-48) carbapenemases; however, it has no activity on MBL (VIM, NDM and IMP) or against the Acinetobacter baumannii complex.59
Ceftazidime, on the other hand, is a drug administered as an intermittent infusion that is not metabolized, with a pharmacodynamic effect that is independent of concentration, a half-life of 1.7-2 hours and a protein binding percentage of 17%; its elimination route is exclusively renal. This drug requires a dosage adjustment to maintain the recommended 4:1 ratio of ceftazidime: avibactam. Its most frequent side effects are abdominal pain, vomiting, diarrhea, headache, and infusion site reactions.60-70 (Tables 4 and 5).
In a meta-analysis involving 11 studies, Onorato et al.71 compared the efficacy of ceftazidime/avibactam as monotherapy and as combination therapy in carbapenem-resistant CPE and P. aeruginosa infections in 396 patients (202 with combination therapy and 194 with monotherapy), finding a mortality rate of 38.1% for combination therapy and 30.9% for monotherapy (RR: 1.18; 95%CI: 0.88-1.58). The researchers found no significant differences in the two groups and the microbiological cure rates were similar (64.9% for combination therapy and 63.4% for monotherapy; RR: 1.04, 95%CI: 0.85-1.28). Based on these results, the study suggested that the use of ceftazidime/avibactam in monotherapy or combination therapy for infections caused by CPE could show a similar effect on mortality and microbiological cure rates, although further research is still required.
On the other hand, Shields et al.72 conducted an observational study of 37 patients with CPE infection treated with ceftazidime/avibactam and reported that the 30-day survival was 76% and the microbiological failure rate was 27%.
In 2018, van-Duin et al.73) published a study comparing the outcomes of 38 patients treated with ceftazidime/ avibactam and 99 patients treated with colistin and found that the 30-day mortality rate was 8% for the former and 33% for the latter. Similarly, using an analysis of disposition at 30 days, that same study found that patients treated with ceftazidime/avibactam had an inverse probability of treatment weighting-adjusted probability of a better outcome of 64%.
The following year, Tumbarello et al.74 conducted a study of 208 individuals with carbapenem-resistant K. pneumoniae infections, 104 treated with rescue regimens containing ceftazidime/avibactam (cases) and 104 with alternative rescue regimens (controls). The therapy was administered for 14 days, finding that the 30-day mortality rate was 36.5% for the case group and 55.7% for the control group.
Between 2015 and 2019, Jorgensen et al.75 conducted a multicenter retrospective cohort study at 6 U.S. medical centers involving 203 adult patients who received ceftazidime/avibactam treatment for multidrug-resistant germ infections, including CPE and P. aeruginosa. They reported that the most frequent sources of infection were respiratory (37.4%), urinary (19.7%) and intra-abdominal (18.7%), that blood cultures were positive in 22 (10.8%) patients, and that clinical failure, 30-day mortality and 30-day recurrence occurred in 59 (29.1%), 35 (17.2%), and 12 (5.9%) patients, respectively. This author also established that primary bacteremia and respiratory tract infection were the factors most associated with clinical failure (OR: 2.27 and OR: 1.23, respectively) and that initiation of ceftazidime/avibactam within 48 hours of infection onset was associated with better outcomes (OR: 0.4). It should be noted that 17 patients (8.4%) experienced potential drug-related adverse effects: 10 acute renal failure, 3 C. difficile infection, 2 skin rashes, 1 gastrointestinal intolerance, and 1 neutropenia.
Between March 2015 and April 2016, King et al.76 conducted a multicenter retrospective cohort study of 60 patients with CPE infection treated at 9 U.S. healthcare centers with ceftazidime/avibactam and found that there was a high prevalence of acute illness: 59% of patients were in the ICU at the time of treatment, 38% required mechanical ventilation, and 21% required vasopressors. In this study, the overall mortality rate was 32%, being higher in patients with pneumonia and in those who required admission to the ICU (46%). No significant differences were found in hospital mortality rates for patients on combination therapy versus patients on ceftazidime/avibactam monotherapy, or for patients with bacteremia versus patients without bacteremia.
Data on the efficacy of ceftazidime/avibactam in critically-ill and mechanically ventilated patients are limited; however, the retrospective cohort study by Tsolaki et al.77 conducted in 2 ICUs in Greece, compared the outcomes of 41 patients who received ceftazidime/avibactam with 36 patients who received other antibiotics to treat CPE infections and found that microbiological eradication was achieved in 94.3% and 67.7% of these patients, respectively (p=0.021), and clinical cure was observed in 80.5% and 52.8%, respectively (p=0.01). Results were similar in the bacteremia subgroups and 28-day survival was 85.4% for patients treated with ceftazidime/avibactam and 61.1% for the others (p=0.035); relapses occurred in 2 and 12 patients in each group, respectively (p=0.042). No significant side effects were reported in this study and the authors concluded that a ceftazidime/avibactam regimen is more effective than other available antibiotic agents for the treatment of CPE infections in ICU patients requiring mechanical ventilation.77
In 2019, Alraddadi et al.78 published a retrospective cohort study conducted between January 2017 and August 2018 in which they compared two groups of patients with CPE infection; the first group (n = 10) was treated with ceftazidime/avibactam and the second group (n=28) received other agents. The authors found that the 30-day all-cause mortality rate was 50% and 57%, respectively, while clinical remission was achieved in 80% and 53%, respectively.
Similarly, observational studies such as those by Bowers et al.79 and Falcone et al.80 compared the mortality outcome in treatments with ceftazidime/avibactam with that of other therapies in patients with urinary tract infection, nosocomial pneumonia, and intra-abdominal and bloodstream infections, reporting lower mortality rates with this first management protocol.
Buckman et al.60 conducted a study in which they evaluated the chemistry, pharmacodynamics, pharmacokinetics, and metabolism of ceftazidime/avibactam for the treatment of complicated intra-abdominal infections. They concluded that, in combination with metronidazole, it is a viable option due to its broad action against ESBL-producing Gram-negative bacteria and thus may be used as an alternative to carbapenems.
In 2020, Tamma et al.81 published a guideline for the treatment of Gram-negative bacterial infections; nevertheless, the methodology of the document is not clear, and the recommendations made lack sufficient scientific evidence to properly support them. Furthermore, it includes drugs that are not available in Colombia, such as meropenem/vaborbactam and imipenem/relebac-tam, and therefore this guideline cannot be applied in the country.
It is worth mentioning that, as demonstrated by Appel etal.,82 resistance to carbapenems among enterobacteria is associated with a susceptibility to ceftazidime/avibactam, which in Colombia ranges from 68.6% to 81%.
As noted above, to date, there are no clinical trials comparing the use of ceftazidime/avibactam with other therapies for the treatment of CPE infections.
Tigecycline
It is a tetracycline derivative that lacks activity against P. aeruginosa (Proteus spp., Providencia spp. and Morganella spp.) as it is intrinsically resistant to this antibiotic. It is used to treat CPE infections, but its clinical efficacy remains controversial.83
Ni et al.83 conducted a systematic review and meta-analysis comparing the efficacy and safety of tigecycline in the treatment of CPE infections with other antimicrobial agents and evaluated whether combination therapy and high-dose regimens are beneficial. The authors included 21 controlled studies and 5 single-arm studies and found that the efficacy of this drug is similar to that of other antibiotics for these types of infections and that tigecycline combination therapy and high-dose regimens may be more effective than monotherapy and standard-dose regimens, respectively. Likewise, Rodrigues et al.,84 based on a systematic review of the literature, concluded that the efficacy of tigecycline in monotherapy may be similar to other antimicrobial options in adult patients with skin and soft tissue infections and that it should be considered especially as adjunctive therapy in patients with polymicrobial infections.
Moreover, Osorio et al.,85 in a study in which they evaluated the available evidence to generate recommendations regarding the efficacy and safety of tigecycline in adults with complicated intra-abdominal infection, found that monotherapy with tigecycline has the same efficacy and safety as other antimicrobial therapeutic options and does not increase mortality compared to other antibiotics.
Additionally, pharmacological modeling studies have considered the use of higher doses to improve the pharmacokinetic/pharmacodynamic relationship of the drug. For example, Xia & Jiang86 conducted a study to determine the safety and efficacy of tigecycline in elderly patients with multidrug-resistant bacterial infections (51 received high doses and 106 received conventional doses) and found that, compared to conventional doses, high doses were associated with better clinical effectiveness (58.8% vs. 34%; p=0.003) and a higher percentage of microbiological eradication (41.2% vs. 23.6%; p=0.023).
Although nausea, vomiting, and diarrhea are the most frequently reported adverse effects of tigecycline, it has been recently established that this drug can cause acute pancreatitis. This effect was analyzed by Hung et al.87 who concluded that being aware of this adverse effect is essential to promote timely and adequate treatment of pancreatitis, including drug discontinuation; therefore, treating physicians should monitor the symptoms of abdominal pain during treatment with tigecycline. Similarly, since tigecycline-induced pancreatitis is still considered a rare phenomenon, the authors recommended further research focused on identifying the mechanism leading to this adverse reaction.
Aminoglycosides
This is a group of bacteriostatic agents that have been used both in monotherapy and in combination with other drugs. They have higher urinary K. pneumoniae clearance rates than tigecycline and polymyxin B.88) van Duin et al.89 studied a cohort of 157 patients with urinary tract infection caused by K. pneumoniae KPC-producing strains, with a sensitivity close to 85%, finding a lower probability of failure when compared to colistin, TMP/ SMX, or fosfomycin.
Nephrotoxicity and ototoxicity caused by aminoglycosides have been established to be 15-50% and 10%, respectively. It has also been found that resistance to these drugs is mediated by the activity of their modifying enzymes and the ribosomal protection of rRNA methyltransferases (ArmA, RmtA, etc.) found mainly in NDM-producing bacteria.90
Fosfomycin sodium
It is a broad-spectrum antibiotic. The literature review conducted for the preparation of this CPG did not find any study with sufficient samples of individuals to compare the outcomes of patients treated with this drug versus other equivalent drugs.
Fosfomycin sodium is not considered the first choice of the treatment for severe CPE infections if other active drugs are available. Furthermore, resistance to this antibiotic has been described in 5% of isolates, even when used in combination with other drugs for infections caused by KPC-producing Enterobacterales. Its toxicity is mainly associated with a high sodium load (14 mEq/g, corresponding to 350 mEq/day with doses of 24g), which has been reported in 15-30% of patients.91
Table 6 Adjustment of fosfomycin sodium depending on renal function.
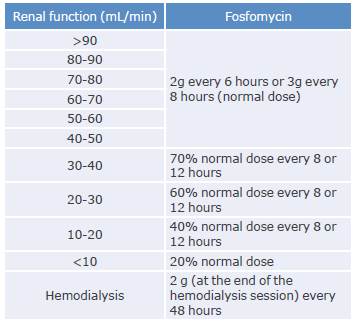
Source: Elaboration based on Cunha & Cunha.58
Treatment with fosfomycin sodium should be adjusted to the patients' renal function, as described in Table 6.
Polymyxins
They are cationic polypeptide antibiotics that have long been considered the last resort for the treatment of infections caused by multidrug-resistant Gram-negative bacteria such as CPE. According to Rodríguez-Baño et al.,53 these drugs are effective against enterobacteria, except for Proteus spp., Serratia spp., Morganella spp., and Providencia spp.
In 2017, Zusman et al.92 published a systematic review that included 22 studies to examine the efficacy of polymyxin-based monotherapy versus combination therapy. In this study, the authors found that 30-day mortality was significantly higher with monotherapy (OR=1.58; 95%CI: 1.03-2.42) compared to combination therapy with tigecycline, aminoglycosides, or fosfomycin.
Zarkotou et al.93 conducted a study that evaluated risk factors for mortality in bloodstream infections caused by KPC and found that overall mortality was 52.8% and infection-related mortality was 34%. They also established that the main predictors of infection-related mortality were APACHE II score at infection onset, advanced age and inadequate antimicrobial treatment, the latter being the only modifiable variable that could be used to improve outcomes. Therefore, the authors concluded that, in addition to implementing infection control strategies, it is critical to identify patients at risk for adverse outcomes and ensure effective evidence-based treatment.
In a retrospective cohort study of 41 patients with Klebsiella pneumoniae KPC-producing bacteria, Qureshi et al.91 found an overall 28-day crude mortality rate of 39% and, using a multivariate analysis, established that combination therapy as definitive therapy remained an independent predictor of survival (OR: 0.07, 95%CI: 0.009-0.71, p=0.02).91
As stated by Tsuji et al.94 in their consensus, the recommendation of using polymyxins for CPE infections is based on the results of some analytical studies (cohorts, cases, and controls) and observational studies (case series) in which there is a significant risk of bias, since there are no clinical trials that establish which is the most appropriate use of this drug: monotherapy or combined therapy.
Additionally, there is considerable debate on the appropriate usage of polymyxins (colistin or polymyxin B) in terms of the identification of their in vitro susceptibility95 and pharmacokinetics, especially in individuals with acute infections.96 In this regard, Osorio et al.,97 citing Abdelraouf et al., suggest that administering polymyxin B every six hours may increase the severity and earlier onset of associated nephrotoxicity, and that administration of a single dose per day equivalent to the amount that would be administered daily every 6 hours would decrease the risk of developing nephrotoxicity without affecting the bacteriostatic activity of the drug.97
In summary, data from clinical studies suggest that polymyxins should not be used as monotherapy in CPE infections and should only be considered as alternatives to available regimens. However, it should be stressed that Tsuji et al.94 published an extensive guideline on the use of polymyxins in collaboration with several specialized medical societies.
The dosage of this drug depends on the polymyxin to be used. Miglis etal.,96 in a population pharmacokinetics study, suggest that a weight-based loading dose and a fixed maintenance dosing strategy, i.e., weight-independent, of polymyxin B may maximize its efficacy and balance toxicity issues for most patients.
Regarding colistin, Tsuji et al.94 recommend initiating intravenous therapy with a loading dose of 300 mg (~9 million IU) infused over half an hour to 1 hour and administering the first maintenance dose 12 to 24 hours later.
Table 7 shows the dosage adjustment of colistin based on renal function.
Table 7 Dosage adjustment of colistin based on renal function.
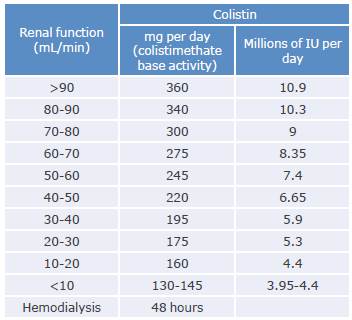
Source: Elaboration based on Cunha & Cunha.58
Carbapenems
These are broad-spectrum antibiotics that must be administered intravenously and include imipenem, meropenem, ertapenem, and doripenem.
This type of drug has also been extensively studied. For example, Kuti et al.98 conducted a study to compare the pharmacodynamic target attainment rates of various meropenem dosing regimens when the infusion is prolonged by more than 3 hours compared to the traditional 30-minute infusion. They found that in the case of mild infections caused by Enterobacteriaceae, prolonging the meropenem infusion by 3 hours allows using a lower dose (500mg prolonged infusion every 8 hours) or increasing the interval between doses (1 000mg prolonged infusion every 12 hours).
In turn, Daikos et al.99 suggest that the therapeutic efficacy of carbapenems against KPC isolates with MIC ≤4 mg/L increases when these agents are administered in combination with another active antibiotic. However, there is still controversy regarding the efficacy of these drugs in monotherapy, as studies published before 2010 indicate that the frequency of treatment failure could be higher compared to their use in combination therapy.
Based on the information presented here, adding meropenem to the treatment may be considered if the isolate has a MIC ≤8 μg/mL, provided that other in vitro active drugs in monotherapy are not acceptable for treating the specific source of infection, especially if the other combinations pose a high risk of toxicity.
Finally, it should be noted that there is no evidence of CPE mediated by the production of MBL, OXA-48 or any other resistance mechanism. The use of carbapenems may facilitate the emergence of higher levels of resistance to this group of drugs or maintain endemicity in countries such as Colombia.
Double-carbapenem therapy
Carbapenems have a broad spectrum of antibacterial activity and play an extremely important role in the treatment of serious infections; however, antimicrobial treatment options to combat carbapenem-resistant Gram-negative bacteria are limited.
In this regard, and taking into account that many combination therapies have shown improved survival and reduced mortality rates compared to monotherapy regimens, Li et al.100 published a study in which they compared the efficacy and safety of double-carbapenem therapy with other antibiotics for the treatment of multidrug-resistant Gram-negative bacterial infections, finding that this modality was as effective as other antibiotics in this context and could therefore be considered as a therapeutic option in patients with these types of infections.
Implementation, applicability, management indicators, and updating of the guidelines
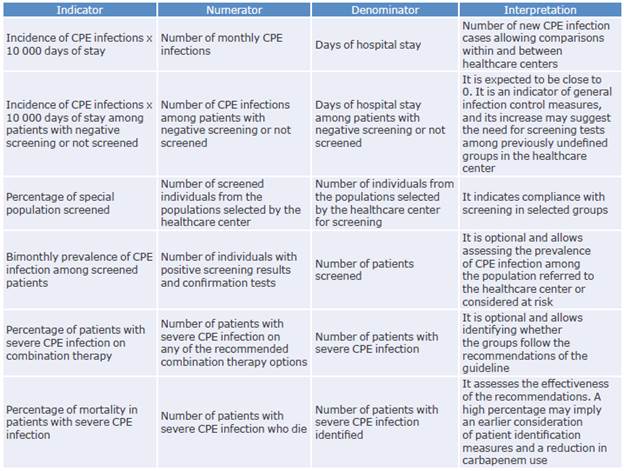
An important element to consider when implementing a CPG is the generation of indicators to monitor its usefulness and performance. In this sense, some indicators that could allow the evaluation of this guideline and generate institutional improvement plans are proposed below.
It is worth mentioning that one of the barriers to access and implementation of this guideline is the inequity of the Colombian health system, as the differences between the commercial values of the diagnostic and treatment options can result in significant restrictions on the acquisition, access, and use of the technology and drugs available in the country. Moreover, as mentioned above, there are no extensive studies in Colombia on the cost-effectiveness of diagnosis and treatment of CPE infections, so research is needed to define the most effective strategies considering the current health system.
Also, this guideline is expected to be updated within the following 5 years or sooner if new evidence or novel antimicrobials with activity against CPE become available in the country.
Table 8 presents the management indicators proposed for the implementation and follow-up of this CPG.