Introduction
Vasoactive intestinal peptide-secreting tumor or VIPoma is a rare functional pancreatic neuroendocrine tumor (F-PNET) characterized by increased production of vasoactive intestinal peptide (VIP), which was first described in 1958 by Verner & Morrison.1
Because of its clinical manifestations, this type of cancer is also known as WDHA syndrome (watery diarrhea, hypokalemia and achlorhydria) or pancreatic cholera.2 VIPomas are usually located in the pancreas and have a high risk of metastasis in up to 80% of patients at the time of diagnosis, especially to the liver and lymph nodes.3,4 Similarly, an association between VIPoma and multiple endocrine neoplasia type 1 (MEN-1) syndrome has been found, although most cases are sporadic.1,5
The case of a woman with a VIPoma without metastasis is presented here to emphasize the importance of suspecting this diagnosis in patients with chronic secretory diarrhea in the absence of infections.
Case presentation
A 61-year-old mestizo woman, with a history of controlled primary pulmonary hypertension and no relevant family history, attended the emergency department of a tertiary care hospital in Medellin, Colombia, due to symptoms consisting of chronic secretory diarrhea (7 stools per day that did not yield with fasting) associated with weight loss of 12kg (66kg at the initial consultation and 79kg before the onset of symptoms), abdominal pain, facial flushing, asthenia and adynamia for 7 months. The patient, who had undergone an outpatient colonoscopy four days before admission that reported mixed hemorrhoids and mucosal prolapse, was treated with rifaximin, antispasmodics, and oral rehydration solution without improvement.
On admission, the patient was dehydrated, drowsy, disoriented and had hypoactive delirium. Laboratory tests were performed, finding moderate hypokalemia (2.74 mEq/L), moderate hyponatremia (126 mEq/L), no hy-perlactatemia (0.81 mmol/L), metabolic acidosis (pH: 7.19, HCO3: 6 mmol/L), normokalemia (10.1 mg/dL), normoglycemia (98.5 mg/dL), and acute kidney injury (BUN: 65.1 mg/dL, Cr: 6.77 mg/dL). Treatment was started with rifaximin 550mg every 12 hours for three days, loperamide, and intravenous fluids; information on the dosage used for the last two medications could not be retrieved from her medical records. Two days after admission, and due to clinical deterioration, the patient was transferred to the intensive care unit (ICU), where she remained for 3 days until she stabilized (her hydroelectrolytic disorder and kidney failure improved, although the diarrhea persisted). She was then transferred to the general floor, where she was tested for bacteria, helminths, protozoa, HIV and Clostridium difficile infections, all of which were ruled out.
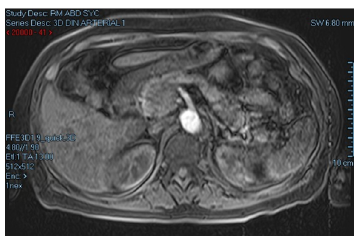
An abdominal magnetic resonance imaging (MRI) was performed on the second day of her stay on the general floor, revealing a mass of 33x30mm between the head and the uncinate process of the pancreas, with no evidence of metastasis (Figure 1). Due to the high suspicion of a neuroendocrine tumor, 6 days after admission a chromogranin A (CgA) measurement was requested, obtaining a value of 477 ug/L (0-100), as well as a 5-HIAA urine test, the result of which was not reported.
Source: Document obtained during the course of the study.
Figure 1 Abdominal MRI showing mass between the head and the uncinate process of the pancreas.
In view of the clinical and laboratory findings, the patient was discharged 15 days after admission, and it was decided that the management would be surgical and ambulatory (hepatobiliary surgery). However, three days after discharge, the patient was admitted to the emergency department of another (also tertiary) care center due to the persistence of the clinical signs described above and altered state of consciousness.
Upon admission to this second hospital, the following vital signs were found: blood pressure of 98/64 mmHg, heart rate of 104 bpm, and respiratory rate of 26 rpm. In addition, the patient was afebrile and without hypoxemia, and the hydroelectrolytic disorder recurred with mild hypokalemia (3.3 mEq/L), moderate hyponatremia (125 mEq/L), hyperlactatemia (22.4 mmol/L), metabolic acidosis (pH: 7.19, HCO3: 6 mmol/L), normokalemia (10 mg/dL), and pre-renal acute kidney injury (BUN: 64 mg/dL, Cr: 2.76 mg/dL). Considering these findings, the patient was initially admitted to the special care unit before being transferred to the intensive care unit the following day due to worsening of symptoms.
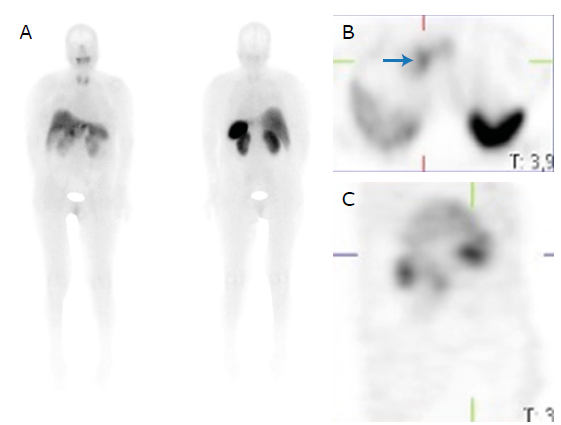
On the third day of admission, and given the high suspicion of a VIPoma, a somatostatin receptor scintigraphy was performed (Figure 2), which showed a lesion proximal to the pancreatic head with somatostatin receptor expression; therefore, management with subcutaneous octreotide 100ug every 8 hours was immediately started, resulting in complete resolution of the diarrhea within 24 hours. Due to the patient's improvement, 4 days after admission to the ICU, she was transferred to the general floor.
Source: Document obtained during the course of the study.
Figure 2 Somatostatin receptor scintigraphy showing uptake at the level of the pancreatic head (blue arrow) and no evidence of metastatic hyperenhancement. A) Anterior and posterior plane; B) Axial plane; C) Sagittal plane.
Thirteen days after admission, the patient had the mass removed by Whipple procedure, with no complications. Serum VIP levels were 930 pg/mL (RV<75), and the pathology report revealed a solid, encapsulated mass with less than 4 mitoses per 10 high-power fields and positive for synaptophysin, (3-catenin, and pancreatic polypeptide. Ki-67 was 3-5% and no metastases to 7 nodes were evident, confirming a grade 2 pancreatic neuroendocrine tumor (PNET).
Twenty days after admission, she was discharged in good general conditions and without permanent pharmacological treatment. Follow-up was scheduled and in the last appointment, which took place two years after the procedure, the patient was asymptomatic and without evidence of residual lesions or metastasis according to recent MRI of the abdomen and pelvis.
Discussion
PNETs have an estimated annual incidence of 1 to 5 cases per million inhabitants, with non-functional PNETs being the most frequent, followed by insulinomas.
VIPoma is a rare type of F-PNET6 that was first reported in 1958 by Verner & Morrison1 in two patients with refractory diarrhea, hypokalemia, and vacuolar nephropathy. These tumors are characterized by persistent diarrhea, even during fasting and with a volume of feces that may exceed 3 000 mL/day, as well as hypokalemia, hypochlorhydria, hyperglycemia and hypercalcemia that do not improve with time and the establishment of an adequate treatment for hydroelectrolytic disorder.
Since neuroendocrine neoplasms are considered malignant, the World Health Organization designed a three-level classification tool for these tumors based on proliferation markers (G1, G2, G3), either KI-67 or mitotic index, which is very useful for estimating patient survival.7 According to this classification, G1, G2 and G3 PNETs are characterized by Ki-67 <3%, 3-20%, and >20, respectively, and a mitotic index <2 (2mm2), 2-20 (2mm2) and >20 (2mm2), respectively.7
VIPomas usually occur in patients between the ages of 30 and 50, and most (90%) originate in the pancreas and are usually malignant. 10-15% of extra-pancreatic cases (colon, lung, liver, or sympathetic chain) originate in the neuroendocrine cells of the intestinal mucosa and along the sympathetic chains, with ganglioneuroblastomas being the most frequent in children,5,8,9 but they can also emerge from pluripotent progenitor cells that develop neuroendocrine characteristics. The pancreatic tail is the most frequent location of VIPomas (75%).10
It should be noted that VIP is a 28-amino acid neuropeptide that acts as a neuromodulator and neurotransmitter, and is normally expressed in the central nervous system, urogenital tract, respiratory tract and gastrointestinal system.11 It is released in response to gastric distension to fulfill its secretory and vasodilator function, and it stimulates the relaxation of the vascular and non-vascular smooth muscle by stimulating cyclic adenosine monophosphate in enterocytes, generating an increase in the release of electrolytes, especially bicarbonate and potassium, which are secreted along with water into the intestinal lumen. VIP also inhibits gastric secretion, stimulates bone resorption, and promotes gluconeogenesis and hyperglycemia.12
In pathological conditions, VIP is released in the absence of stimulation and causes the appearance of chronic secretive diarrhea and major gastric losses of potassium, bicarbonate, and chlorine, which translates into hypokalemia and hypochloremia. In addition, metabolic acidosis, renal failure (as a result of hypovolemia), weight loss, abdominal pain, nausea, and asthenia, hyperglycemia, hypercalcemia and facial flushing may also occur in these cases.13
The biochemical diagnosis of VIPomas is made by establishing whether the VIP concentration is increased; then, the location of the lesion is established using imaging scans.14 The difference with other neuroendocrine tumors is determined based on clinical presentation and laboratory alterations indicating the need to measure other hormones such as gastric pH, gastrin, insulin, or glucagon when Zollinger-Ellison syndrome, insulinoma or glucagonoma are suspected, respectively.6 The patient reported here did not have any symptoms associated with peptic ulcer disease or changes in blood glucose levels, so no studies were conducted to rule out the differential diagnoses mentioned.
Although VIPomas are rare tumors, it is important to suspect their presence in patients with chronic secretory diarrhea that does not improve with fasting, in whom infectious causes such as HIV, C. difficile and Vibrio cholerae have been ruled out, and in patients with electrolyte disorders.
Early diagnosis of VIPomas is essential to prevent the natural progression of the disease and associated complications, such as dehydration, metabolic acidosis, and kidney failure, which were present in the reported patient, and in whom the symptoms started 7 months before the diagnosis was made. In this regard, it should be noted that the average time for the diagnosis of this type of cancer is 15 months after the onset of symptoms due to lack of suspicion.
Although no metastases were found in the present case, it should be borne in mind that up to 80% of patients with VIPoma present with metastatic involvement at the time of biochemical confirmation, and that it occurs mainly in the liver and lymph nodes, having a negative impact on the survival and prognosis of patients.2
The median survival for patients with VIPoma is 7.9 years;15 however, poor prognosis is associated with the histologic grade of VIPoma, staging, and presence of metastases.6 Absence of metastases, age <50 years, surgical resection, and stage I/II disease are good prognostic factors.
The initial diagnostic approach to the reported patient was based on the measurement of CgA after the imaging finding in the pancreas; it is a glycoprotein contained in the vesicles of neuroendocrine cells and secreted by almost all neuroendocrine tumors that is frequently elevated in patients with PNET. This hormone is a useful marker for the prognosis of this type of tumors since, as stated by Landry et al.,16 citing Boudreaux et al., it has sensitivity and specificity that vary between 70% and 100% depending on the extent of the disease.16
Elevated CgA levels have been associated with rapid tumor progression and short patient survival, so its measurement may be a useful tool for follow-up; however, its evaluation in patients taking somatostatin analogues should be cautious because these drugs affect their production and release.16,17 Furthermore, false positive results may be obtained, particularly due to the frequent use of proton pump inhibitors, which must be suspended one week before measurement.18,19
Similarly, pancreastatin is a useful marker in metastatic tumors that, while not influenced by as many conditions as CgA, is elevated when renal failure occurs and medications that increase glucose levels are taken; it is worth mentioning that this marker is not routinely available in Colombia.16,17
Most PNETs are highly vascular and very sensitive to contrast-enhanced imaging, either CT or MRI (sensitivity of up to 92% is reported),20 especially when visualized in arterial phase. However, tumor size is critical in tests such as CT: sensitivity <10% has been reported in sizes <1cm, while sensitivity is close to 100% in sizes >3cm.20
Although VIPoma is usually diagnosed using CT scans or MRI, other diagnostic means such as endoscopic ultrasonography, somatostatin receptor scintigraphy, or positron emission tomography (PET) with gallium 68-DOTATATE are sometimes necessary, especially when the primary site is unknown or metastases are sought.21 It should be borne in mind that PET with gallium 68-DOTATATE is very useful in cases in which the primary site is unknown,22-24 although its availability in Colombia is scarce. The reported patient underwent MRI and somatostatin receptor scintigraphy, which allowed detecting the pancreatic lesion and the absence of metastasis.
Since the quality of life of patients can be compromised by VIPoma and because its presence can even lead to life-threatening situations, symptomatic treatment should be initiated even before the exact location of the tumor is known, i.e., as soon as analytical tests suggest its presence. An alternative for symptomatic management is the use of somatostatin analogues, which bind mainly to somatostatin receptor 2, inhibiting VIP release and antagonizing the effects of tumor growth factors.13,25,26
In Colombia, the available somatostatin analogues are octreotide and lanreotide, which act on the receptor 2 13,27 and allow controlling the overproduction of VIP in a transitory manner. Both have extended-release formulations and their adverse effects are usually mild and include abdominal pain, nausea, and steatorrhea; asymptomatic cholelithiasis has also been reported due to their chronic use, but it should not be operated on.
Due to low cure rates, and in order to identify recurrences early, most patients with VIPomas should be followed up on indefinitely with CgA or VIP measurements, although information in this regard is scarce and this would make more sense in case of symptom recurrence. Nevertheless, the most recent follow-up guidelines for neuroendocrine tumors suggest performing imaging studies (CT or MRI) every year for the first 3 years and then every 1-2 years for a total of 10 years.28
Conclusions
VIPomas are rare neuroendocrine tumors that secrete VIP and are characterized by symptoms consisting of chronic diarrhea, hypokalemia and aclorhydria. Given the severity of the associated hydroelectrolytic disorders, these neoplasms can lead to a fatal outcome for the patient, so, despite their low prevalence, they should be suspected as a differential diagnosis in patients with chronic secretory diarrhea and electrolyte disturbances in whom infectious causes have been ruled out.
Due to the nature of the disease, the rate of metastasis at the time of VIPoma diagnosis may reach 80%, which translates into worse prognosis and survival of patients, hence the importance of having a high rate of suspicion of these tumors that allows for an early diagnosis. The diagnostic approach should include imaging findings of the tumor and confirmation of elevated VIP levels. Although location is usually pancreatic, in cases where the primary site is unknown, the use of functional images such as gallium 68-DOTATATE PET scan may be very useful.
Treatment of symptoms begins with fluid replacement and correction of electrolyte disorders, measures that should be established even when the imaging report is not available because these variables put the patient's life at risk. After checking somatostatin receptor uptake, treatment of symptoms with short-acting octreotide may be initiated, but it should be borne in mind that although this medication is effective for the management of diarrhea, its response is transient and a definitive treatment, which corresponds to surgical resection of the tumor, should be sought.
In summary, late diagnosis of VIPoma is associated with poor of quality of life, severe complications, and high prevalence of metastasis, so it should be suspected in patients with chronic secretory diarrhea that is not caused by infections, because better survival rates can be achieved in these patients if diagnosed on time and timely treatment is initiated.















