Introduction
The number of obesity cases has been rapidly increasing worldwide.1 It is one of the most common metabolic diseases, even to the point of being considered an epidemy2 and one of the most serious public health issues.3 Furthermore, obesity is related to a number of comorbidities such as diabetes, hypertension, increased cardiovascular risk, as well as social and psychological disturbances4 and postural and osteoarticular disorders,5 posing a heavy economic burden on public health systems.
Obesity is typically diagnosed and classified based on the body mass index (BMI). BMI values equal to 40 kg/m3 or higher indicate grade III obesity or morbid obesity.6 In Brazil, the prevalence of severe obesity in women has increased significantly, going from 1.3% in 2006 to 1.9% in 2017.7
Sarcopenia, which is associated with a decrease in muscle mass and, consequently, loss of muscle strength and functional autonomy, can result in adverse health outcomes8 and interfere with the daily life of obese patients due to their low level of physical activity. In this context, interventions focused on treating obesity based only on anthropometric values without considering muscle mass may be related to some risks,4 especially in individuals with sarcopenic obesity.9 In such a population, the most appropriate way to analyze body composition and body compartments is bioelectrical impedance (BIA), a method that allows studying body composition in fractions10 and providing a detailed assessment, mainly regarding the regional distribution of fat and lean mass, besides assisting in the diagnosis of sarcopenia.11 This method is considered to be simple, safe and easy to use, characteristics that are relevant for use on an outpatient basis.12
The compartment models of body composition, by providing more reliable and individualized monitoring, may assist in nutritional guidance and the planning of physical exercise programs, especially in cases of severe obesity. Thus, the objective this study was to analyze the body characteristics of morbidly obese (class III) women referred to bariatric surgery using the compartment model of body composition analysis.
Materials and methods
Cross-sectional study conducted in morbidly obese women referred to the Bariatric Surgery Program of the Clementino Fraga Filho University Hospital, from the Federal University of Rio de Janeiro (PROCIBA / HUCFF-UFRJ) in 2017. This program, which has a multidisciplinary team that includes doctors, physiotherapists, physical education professionals and psychologists, among others, recruits individuals with grade III obesity for clinical analysis and subsequent referral for bariatric surgery.
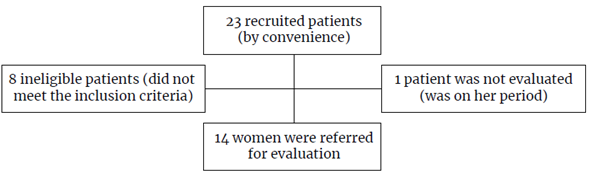
A total of 14 morbidly obese women aged between 25 and 51 years who were undergoing clinical and physical therapy, as well as physical tests prior to bariatric surgery were recruited through convenience sampling. The selected individuals were not in the menopause period and had not engaged in any physical activity before taking the tests. Patients who were pregnant, used a pacemaker, were on diuretic medication or reported previous bariatric surgery were not included in the sample, as seen in the study flowchart below (Figure 1).
The study was carried out in accordance with the ethical principles for conducting biomedical research involving human subjects of the Declaration of Helsinki13 and was approved by the Ethics and Research Committee of the Federal University of Rio de Janeiro on September 9, 2013, as stated in the CAAE (Certificado de apresentação para Apreciação Ética) No. 13524013.7.0000.5257, CEO-HUCFF process 132-13, group III. The participation in the study was voluntary and all individuals signed an informed consent letter authorizing data collection and the development of the study.
Initially, data on demographic, comorbidities and use of medications were collected, along with information about the last menstrual period and their physical activity levels, because only patients who did not perform physical exercises were accepted. Then, anthropometric data were collected using a fixed Sanny™ stadiometer for measuring height with the patient in orthostatic position with legs together, arms along the body, and head parallel to the ground. The measurement was taken during in inspiratory apnea.
Body composition and total body mass (TBM) were determined in the morning using an InBody™ 230 octopolar bioimpedancescale, which measures body composition based on body resistance to an electric current emitted by eight different poles. The test was performed after individuals voluntarily urinated, standing barefoot, wearing no metal objects and as little clothing as possible. Moreover, the patients fasted for four hours and did not consume any alcohol, energy drinks, or caffeine 24 hours before the exam.
They were also around the 14th day of their menstrual cycle, which means they had a lower water retention index.14 To assess bioimpedance, the individuals were instructed to stand in an orthostatic position, facing the scale, with parallel feet slightly apart on the electrodes. Then, data on age, height and sex were entered into the scale. For data collection, the participants held the electrodes attached to the upper part of the scale with both hands.
Body composition assessment through bioimpedance allowed for the analysis of total muscle mass, total and segmental fat mass, total body mass, total body water, BMI, waist-to-hip ratio (WHR), and total and segmental body fat. All data were presented in kilograms (kg) and percentages (%). The gold standard methods for obesity evaluation, such as dual-energy X-ray absorptiometry (DXA) and underwater weighing, were not used in the present study due to their high cost, the need of appropriate evaluator training, and the impossibility of using them in morbidly obese patients.
Sarcopenia was determined using the protocol proposed by Baumgartnert et al.,15 which is based on the sum of the lean muscle mass of the limbs (kg) divided by the square height (m). The cut-off point for women is 5.45 kg/m2. This protocol is intended for the elderly population and was adapted for this study.
Statistical analysis
Data was analyzed using descriptive statistics, including mean, standard deviation, and minimum and maximum values measures for each variable. According to the Kolmogorov-Smirnov test, data had a normal distribution in all variables. Thus, the Pearson's correlation coefficient was used to analyze the correlation between variables, with a significance level of p<0.05.
Results
Participants' age ranged from 26 to 51 years, with a mean of 35.6±6.6 years. Their BMI ranged from 41 to 53 kg/m2, with an average of 46.0±4.0 kg/m2.As for the sarcopenia index, all participants had a minimum value that was greater than the cut-off point of 5.45 kg/m2. Anthropometric and body composition data are depicted in Table 1.
Table 1 Mean, standard deviation (SD), and minimum and maximum values for anthropometry and body composition of the sample.
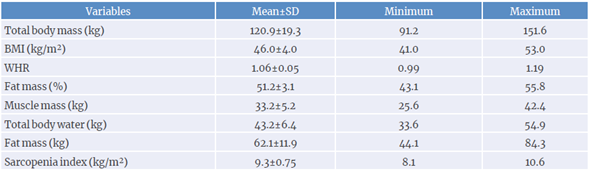
BMI: body mass index; WHR: waist-to-hip ratio.
Source: Own elaboration.
A descriptive analysis of segmented fat mass was also performed (mean, standard deviation, minimum and maximum percentage values), along with the muscle mass, in kilograms. It was found that patients had a higher fat concentration when compared to their body muscle mass. In addition, fat accumulation in the trunk region was higher when compared to fat accumulation in the upper or lower limbs. Table 2 presents such data.
Table 2 Body composition parameters by segment.
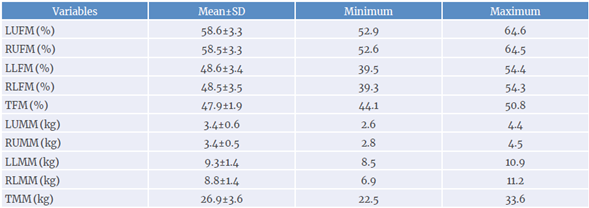
LUFM: left upper limb fat mass; RUFM: right upper limb fat mass; LLFM: left lower limb fat mass; RLFM: right lower limb fat mass; TFM: trunk fat mass; LUMM: left upper limb muscle mass; RUMM: right upper limb muscle mass; LLMM: left lower limb muscle mass; RLMM: right lower limb muscle mass; TMM: trunk muscle mass.
Source: Own elaboration.
In Table 3, it is possible to analyze the correlations between obesity rates and body composition variables. BMI showed a higher correlation with the amount of fat (both in kg and %) than with the WHR (r=0.93, r=0.67 and r=0.55, respectively). Furthermore, total trunk fat (%) showed a positive correlation with total body fat (%) (r=0.76). No patient had sarcopenia and the correlation between muscle mass and the sarcopenia index (r=0.79) demonstrated the importance of taking actions to increase muscle mass to reduce the risk of developing this condition.
Table 3 Correlation between anthropometric and body composition variables.
| BMI | WHR | Sarcopenia index | TBF (%) | TTF (%) | MM | Fat (kg) | TBW | |
|---|---|---|---|---|---|---|---|---|
| BMI | 1 | 0.55* | 0.56* | 0.67* | 0.16 | 0.60* | 0.93* | 0.63* |
| WHR | 0.55* | 1 | -0.44 | 0.60* | 0.51 | 0.18 | 0.57* | 0.20 |
| Sarcopenia index | 0.56* | -0.44 | 1 | -0.55 | -0.59* | 0.79* | 0.56* | 0.78* |
| TBF (%) | 0.67* | 0.60* | -0.55 | 1 | 0.76* | -0.08 | 0.62* | -0.45 |
| TTF (%) | 0.16 | 0.51 | -0.59* | 0.76* | 1 | -0.57* | 0.07 | -0.56* |
| MM | 0.60* | 0.18 | 0.79* | -0.08 | -0.57* | 1 | 0.73* | 0.99* |
| Fat (kg) | 0.93* | 0.57* | 0.56* | 0.62 | 0.07 | 0.73* | 1 | 0.75* |
| TBW | 0.63* | 0.20 | 0.78* | -0.45 | -0.56* | 0.99* | 0.75* | 1 |
BMI: body mass index;WHR: waist-to-hip ratio; TBF (%): total body fat; TTF (%): total trunk fat; MM: muscular mass; TBW: total body water.
*Significant correlation p<0.05
Source: Own elaboration.
Discussion
A higher concentration of fat in the trunk and upper limbs was observed in the sample studied, a population of women with severe obesity during the pre-operative period for bariatric surgery, along with greater muscle mass in the lower limbs. At the same time, BMI showed a stronger correlation with fat content (both in kg and %) than with WHR, while no sarcopenia was observed.
Obesity is commonly diagnosed using the BMI, which is an easy-to-use method that allows establishing increased cardiovascular risk16 and serves as a tool for analyzing the health of a population17 In severe obesity, BMI values are equal to or higher than 40 kg/m2, which denotes a greater risk of mortality and development of chronic diseases.18 However, despite its utility, this index does not allow predicting the amount or distribution of body fat, nor does it differentiate fat mass from lean body mass.19 In the present study, BMI showed a strong correlation with body fat (kg) (r=0.93) and total body fat (%) (r=0.67), but no correlation with fat accumulated in the trunk region.
In this context, Wrzesinski et al.20 state that the way fat is distributed should be considered, as it is linked to a variety of complications. For instance, central obesity increases the risk of mortality21 and is associated with increased arterial stiffness and greater cardiovascular risk, particularly in severe obesity.22
On the other hand, WHR is an anthropometric index widely used in the assessment of obesity and is better correlated with subclinical atherosclerosis than with BMI both in postmenopausal and pre-menopausal women.23 Sahakyan et al.21 also report a greater relationship between the risk of mortality and WHR in comparison with BMI. In the present study, WHR and BMI showed a moderate (r=0.55) and differentiated correlation with anthropometric variables (Table 3). When analyzing the relationship between WHR and body fat amount and distribution, a lower correlation was observed with total body fat (%) (r=0.60) and total fat (kg) (r=0.57), while no relationship with body fat (%) was found in the trunk (r=0.51). Although no correlation was found between WHR and trunk fat (r=0.51), this index performed better than BMI for assessing central obesity.
Schult et al.,24 as well as Parsa & Jahanshahi,25also found a better correlation between WHR and the risk of developing liver cirrhosis than BMI in women in a study of coronary artery disease patients. However, Myint et al.26 reported that both BMI and WHR were good predictors of mortality and cardiovascular disease in both adults and the elderly. Thus, to minimize errors in different population analyses, the use of more than one obesity index would be valuable for correlation analysis and predicting comorbidities when it comes from the concentration and distribution of body fat.27
According to Okorodudu et al.,28 obesity in women is defined as a total body fat value above 30%. In this study, total body fat was 51.2%, clearly above that reference level, ranging from 43.1% to 55.8% (Table 1). In addition, the values for BMI, total body fat (%), total fat (kg), total body mass and total lean mass found in this study are similar to the findings in other studies on severe obesity.29,30
When correlating BMI, WHR and total body fat (%), it is recommended to use different indexes and measurements related to obesity and adiposity, since BMI has limitations for the analysis of fat distribution.31 In the present study, the highest correlations found were between BMI and total fat weight (kg) (r=0.93), and between BMI and total body fat (%) (r=0.67). On the other hand, WHR correlated with total fat weight (kg) (r=0.57) and total body fat (%) (r=0.60).
Regarding sarcopenia, a marker of the decrease in muscle mass, strength and physical performance, it is well known that it impairs performance in daily tasks and physical activity, especially in the elderly;32 as a result, quality of life can be severely compromised in this age group.33 In obese individuals, when excessive body fat compromises physical performance, leading to low muscle function and mass, the condition is defined as sarcopenic obesity.34
According to Saitoh et al.,35 findings such as decreased energy expenditure, hormonal factors, chronic inflammation, and insulin resistance are potential risk factors for the development of sarcopenic obesity. In a study conducted by Santos et al. ,33 the reduction in lower limb mobility and strength were the variables that had the greatest influence on the walking speed and muscle weakness associated with sarcopenic obesity.
Therefore, the loss of muscle mass observed in severe obesity poses a special risk for adverse outcomes.36 In the present study, the sarcopenia index was above the cut-off point of 5.45 kg/m2, which is considered a significant finding since the lower this parameter, the greater the risks for physical health. In this context, Kim et al.37 report that the decrease in body fat and the increase in muscle mass and strength can reduce the risk of surgery for obesity. In addition, there is a possibility of a better hydration level since muscle mass is positively related to total body water.38
As a reference for total body water, Altman & Dittmer-Katz39 suggest a percentage of 50% for total body mass, ranging from 41% to 60% in women aged between 19 and 50 years. In women over the age of 51, the percentage drops to 47%, with an interval between 39% and 57%. In individuals of both sexes with severe obesity, da Silva29 found an average of 28.5% for muscle mass and 37.4% for total body water.
Similarly, in the present study, there was a high correlation (r=0.79) between muscle mass and sarcopenia, suggesting that physical activity may contribute to the reduction of muscle loss and functionality,40 lowering the risks of falling, functional incapacity, and fractures. However, it is worth remembering that such a topic deserves further analysis, as sarcopenia may be associated with a low nutritional status and not only with the functional one.41
Finally, the need for further studies in individuals with grade III obesity is highlighted, not only for treatment guidance, but also for expanding knowledge about the evolution of body composition, particularly in relation to physical training programs and nutritional approaches.
Conclusions
Despite having more muscle mass in the lower limbs and more fat in the trunk and upper limbs, none of the study participants had sarcopenia. On the other hand, BMI had a stronger correlation with both total fat (kg) and TBF% than with WHR. These findings suggest that the assess -ment of obese individuals based only on a single variable, such as BMI or WHR, may hinder the understanding of the obesity profile and the development of individualized treatment strategies for this population.