Introduction
Wilms tumors are the most frequent renal tumor in pediatric population, accounting for 90% of all cases1. In addition, 80% of these tumors occur in children under 5 years of age, with a peak incidence between the ages of 2 and 3 years. 1,2
Cases in which Wilms tumor diagnosis is not clear, whether due to imaging findings or the age range of the patient, are particularly challenging. In this sense, it has been reported that there are several imaging findings that help differentiate tumors of renal origin from those of non-renal origin, such as the "prominent feeding artery sign" and the "beak sign".3 However, there are few works addressing the differentiation of Wilms and non-Wilms tumors based on imaging findings. 4
Age is a relevant variable for differentiating renal tumors in pediatric population. For example, although congenital mesoblastic nephroma occurs in only 3-6% of childhood renal tumor cases, 5 it is the most common kidney neoplasm in children under one year of age, being diagnosed more frequently within the first three months of life (up to 90% of cases). 5 On the other hand, renal cell carcinoma is the second most common kidney cancer in children, but it has a higher incidence during adolescence. 6
The development of Wilms tumors has been usually associated with having nephrogenic rests, 7 congenital disorders (aniridia and hemihypertrophy), 8 and several genetic disorders such as Beckwith-Wiedemann syndrome, Wilms tumor-aniridia syndrome, Denys-Drash syndrome, Perlman syndrome and Bloom syndrome. 9 In this regard, it has been described that in patients with aniridia, Denys-Drash syndrome, Beckwith-Wiedemann Syndrome, and hemihypertrophy, the risk of developing a Wilms tumor is of up to 50%, 40%, 4%, and 3%, respectively. 10
There are several imaging findings characteristic of pediatric renal tumors, including the presence of fatty tissue in angiomyolipomas, calcifications in ossifying renal tumors of infancy or cysts in cystic nephromas. 2,10 In other childhood kidney tumors, such as rhabdoid tumor, a more aggressive cancer that has been associated with the development of posterior fossa tumors, subcapsular fluid collections are usually observed in imaging studies. 11-12
In most cases, pediatric renal tumors are large at the time of diagnosis; for this reason, children have higher rates of radical nephrectomy compared with adults. 13 Therefore, the role of imaging studies in these patients has been limited to surgical planning and metastasis detection. 2,14,15 However, cross-sectional imaging CT scan or MRI provides excellent anatomical detail and has improved the ability to make a more accurate diagnosis of childhood kidney tumors. 16 For example, the use of magnetic resonance imaging was limited to differentiate Wilms tumors from nephrogenic rests and to detect inferior vena cava thrombosis, but now the use of this imaging technique as a tool to study renal tumors has increased. 17,18
In this regard, it has been reported that diffusion-weighted imaging is useful to predict the histological types of Wilms tumors. 19 In fact, low apparent diffusion coefficient (ADC) values have been associated with high-risk histological types, which allows better surgical planning and the personalized selection of chemotherapy. 19
Taking this into account, the main objective of this work was to describe the imaging findings of renal tumors in children. Another objective was to assess the association between imaging findings and histological diagnosis of Wilms tumors versus Non-Wilms tumors, and between imaging features and intraoperative rupture of Wilms tumors, as well as the level of agreement between radiological and histological diagnosis (Wilms vs. Non-Wilms tumor).
Materials and methods
Study type, study population and sample
Cross-sectional study. The study population consisted of all patients with a histo-pathological diagnosis of renal tumor admitted to a children's hospital in Bogotá D.C. (Colombia) between January 2012 and April 2018 who underwent imaging studies at the Radiology Service of the hospital (N=58). Once the medical records, the pathology reports and the radiology reports of the 58 children were reviewed, 11 patients (9 with a Wilms tumor in 10 kidneys, 1 with a congenital mesoblastic nephroma, and 1 with a history of tuberous sclerosis and a complex renal cyst) were excluded due to the absence of reliable data on their imaging studies (i.e., non-availability of images or poor-quality images). Taking this into account, a total of 47 patients aged between 7 days and 14 years were finally included in the study.
Procedures
The following data were obtained from the medical records of the 47 children: demographic information, personal history of disease (e.g., history of Beckwith-Wiedemann syndrome, tuberous sclerosis, among others), and clinical information (presence of abdominal mass, high blood pressure, hematuria, hyperparathyroidism and abdominal pain). Likewise, information on surgical findings was also collected, including the presence of rupture during surgery.
Imaging studies interpretation
Two pediatric radiologists, one in training at the time the study began and another with 17 years of experience, reviewed the ultrasound, computed tomography (CT) scans and magnetic resonance imaging (MRI) of the patients available in the Picture Archiving and Communication System (PACS) of the Radiology Service of the hospital. It should be noted that both radiologists had no prior knowledge of the clinical or epidemiological data of the patients. Tumor size, tumor composition, tumor contrast enhancement, the presence of pseudocapsule, vena cava invasion and evidence of metastasis were evaluated.
Regarding imaging findings, the following variables were considered: tumor localization (right, left kidney or bilateral); tumor composition (cystic, fat, solid, calcified); tumor volume (calculated using the formula for an ellipsoid: diameters in centimeters [longitudinal x anteroposterior x transverse x 0.52]); tumor size (measured in centimeters); tumor enhancement, defined as the increase in tissue density in contrast-enhanced CT scans or MRIs, which can be homogeneous (diffuse) or heterogeneous (patched); presence of bleeding; presence of necrosis; presence of pseudocapsule; and presence of abdominal lymphadenopathy.
In the case of CT scans, cysts were defined as rounded, thin-walled lesions when their density was between 0 and 20 Hounsfield Units (HU); lesions with a density between 30 and 70 HU were defined as solid or soft tissue lesions; lesions with negative density were classified as lesions with fat content; and those with a density >300 HU were defined as calcifications.
The following imaging studies were available at the PACS of the Radiology Service: CT scan (43 patients), ultrasound (19 patients), MRI (8 patients). Five children had the 3 diagnostic modalities, 14 had 2, and 28 had only 1.
After reviewing the imaging studies of each patient, both radiologists, through consensus, reached a diagnosis and classified renal tumors into Wilms and non-Wilms tumors; disagreements were solved with the help of a third radiologist.
Statistical analysis
Means and standard deviations were estimated for continuous variables if the data had a normal distribution (established with the Shapiro-Wilk test), while medians and interquartile ranges were calculated for non-parametric variables. Qualitative variables were expressed using absolute and relative frequencies (percentages).
A univariate logistic regression analysis was performed to determine the association between radiological findings (i.e., tumor composition, enhancement, size, among others) and the histological diagnosis of Wilms and non-Wilms tumors by calculating the respective odds ratios (OR) with a 95% confidence interval. Additionally, a concordance analysis was performed using the Cohen's kappa coefficient to assess the level of agreement between radiological and histological diagnosis (Wilms versus non-Wilms tumor).
Finally, a univariate logistic regression analysis was conducted to establish the association between imaging features and intraoperative Wilms tumor rupture using OR and a 95% confidence interval. Stata statistical software 13 was used for statistical analysis and a level of significance of p<0.05 was considered for all statistical analyses.
Ethical considerations
As medical records and imaging studies of children were reviewed, the study complied with the ethical principles for conducting medical research involving human subjects outlined in the Declaration of Helsinki, 20 as well as the technical, administrative and scientific standards to conduct biomedical research in Colombia set forth in Resolution 8430 of 1993 issued by the Colombian Ministry of Health. 21 In addition, the study was approved by the Institutional Ethics Committee of the Fundación Hospital Pediátrico La Misericordia on November 21, 2017 (Minutes No. 008, CEI-77-17).
Results
Of the 47 patients included in the study, 25 were girls (53.19%), and renal tumor involvement was reported in 57 kidneys (bilateral involvement in 10 kidneys, and isolated involvement of 14 left and 13 right kidneys). According to the histopathology reports, 71.93% (n=41) of the kidneys had Wilms tumors, while the remaining 28.07% (n=16) had non-Wilms tumors. The distribution of tumors was similar in girls and boys: of the 41 Wilms tumors, 21 (51.22%) were reported in girls, and of the 16 non-Wilms tumors, 8 (50%) were described in boys.
In the Wilms tumors group (n=41), the following clinical findings were identified: abdominal mass (68.29%), abdominal pain (44.90%), hematuria (12.19%) and high blood pressure (7.31%). On the other hand, in the non-Wilms tumor group (n=16), the presence of abdominal mass, abdominal pain, hematuria, and high blood pressure were described in 50%, 43.75%, 18.75% and 6.25% of tumors, respectively. There were no patients with hyperparathyroidism.
Regarding the diseases associated with a higher risk of developing renal tumors, three children in the Wilms tumors group had a genetically confirmed diagnosis of Beckwith-Wiedemann syndrome, while in the non-Wilms tumor group one patient had tuberous sclerosis (the kidney tumor in this patient was an angiomyolipoma).
Data on birth weight was available for 23 of the patients with Wilms tumors, and fetal macrosomia was reported in 4 (17.39%) of them. In the non-Wilms tumors group this information was available in 8 children and there were no fetal macrosomia cases.
The average age in the Wilms tumors group was 3.1 years (SD: 2.0), with an age range between 9 months and 9 years, while in the non-Wilms group it was 4.8 years (SD: 5.1), with an age range between 7 days of age and 14 years. Out of the 47 children, 12 were under the age of 1, 25 were aged between 1 year and 5 years, 8 were between 6 and 10 years of age, and 2 were older than 10 years.
Furthermore, in the <1-year group, a total of 13 renal tumors affecting the same number of kidneys were identified (7 Wilms tumors, 3 congenital mesoblastic nephromas, 1 rhabdoid tumor, 1 perirenal neuroblastoma, and 1 immature teratoma). In the 1-5-year-old group, the presence of a Wilms tumor was confirmed in 28 kidneys; in addition, nephroblastomatosis was observed in 2 kidneys. In the 6-10-year-old group, a Wilms tumor was detected in 6 kidneys, there was evidence of leukemic infiltration in 2 kidneys, nephroblastomatosis was evidenced in 2 kidneys, and an angiomyolipoma was found in 1 kidney. Finally, in the >10 years-old group, vascular venous malformations were found in 2 kidneys and there was a case of kidney lymphoma (1 kidney).
Wilms tumors
A total of 41 Wilms tumors were identified, being the most frequent renal tumor in the sample (71.93%). Regarding cancer staging, of the 37 patients with Wilms tumor, 24.32% were classified as stage I, 8.11% as stage II, 37.84% as stage III, 18.92% as stage IV, and 10.81% as stage V. Only 2 children (5.40%) had an unfavorable histological subtype.
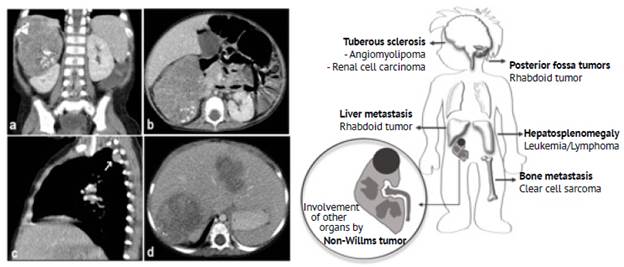
All Wilms tumors were characterized as solid masses (100%), 48.78% were larger than 10 cm, heterogeneous enhancement was observed in 95.12%, necrosis was present in 87.80%, and the presence of pseudocapsule was observed in 82.92%. Figure 1 shows the CT scans of a Wilms tumor in one of the patients included in the study to illustrate the typical features of a Wilms tumor.

Source: Picture Archiving and Communication System of the Radiology Service of the Fundación Hospital Pediátrico La Misericordia. Illustration: own elaboration.
Figure 1 Wilms tumor in a 1-year-old girl. Contrast-enhanced CT scan of the abdomen: axial (a), coronal (b) and sagittal (c) planes. >10 cm right renal solid mass with heterogeneous enhancement and areas of lower density due to necrosis. Pseudocapsule formed by spared renal parenchyma (black arrow on figure 1.a). The characteristics of Wilms tumors are summarized in the illustration
There were 4 cases (10.81%) of bilateral Wilms tumor (stage V). Inferior vena cava invasion and calcifications were observed in 4 (9.75%) and 5 (12.19%) Wilms tumors, respectively. Metastasis at the time of diagnosis was only reported in 7 patients, and all cases were lung metastases. Table 1 summarizes the imaging features of the Wilms and non-Wilms tumors analyzed in the study.
Table 1 Imaging features of Wilms and non-Wilms tumors.
CNS: central nervous system.
Source: Own elaboration based on the data obtained in the study.
In the univariate analysis, a statistically significant association was found between histological diagnosis of Wilms tumor and presence of necrosis (OR:21.6; 95% CI: 4.1-122.8), tumor enhancement (OR:15.17; 95%CI: 2.2-162.3), presence of pseudocapsule (OR:14.57; 95%CI: 3.0-76.9), tumor volume (OR:7.93; 95%CI: 1.0-58.3), tumor size (OR:4.37; 95%CI: 1.7-11.2) and signs of rupture (OR:8.21; 95%CI: 1.0-367). Likewise, having a Wilms tumors was associated with a lower presence of metastasis (OR:0.19; 95%CI: 0.04-0.79). Associations between radiological findings and histological diagnosis of Wilms tumor in the univariate analysis are shown in Table 2.
Non-Wilms tumors
Out of the 57 renal tumors identified, 16 (28.07%) were non-Wilms tumors; of these, 87.50% were characterized as solid masses, 93.75% had a volume <500cc (93.75%), and invasion of the renal collecting system was observed in 6 tumors (37.50%); besides, there was no evidence of tumor enhancement in 4 tumors (25%). There were 6 cases of metastasis at the time of diagnosis. Table 1 shows the imaging features of non-Wilms tumors.
Nephroblastomatosis
Nephroblastomatosis was observed in seven kidneys: there were only nephrogenic rests in 4 kidneys, and the presence of a Wilms tumor was confirmed in the remaining 3. Both intralobar and perilobar nephrogenic rests, as well as multifocal and diffuse involvement were detected.
Congenital mesoblastic nephroma
There were three patients with this type of tumor, all younger than 6 months of age.
Leukemia/Lymphoma
There was one case of leukemic infiltration of the kidney and another case of lymphomatous infiltration of the right kidney. Lack of enhancement and homogeneity of the lesions were the main imaging features in both patients; besides, involvement of other organs was also detected in these two patients.
Rhabdoid tumor
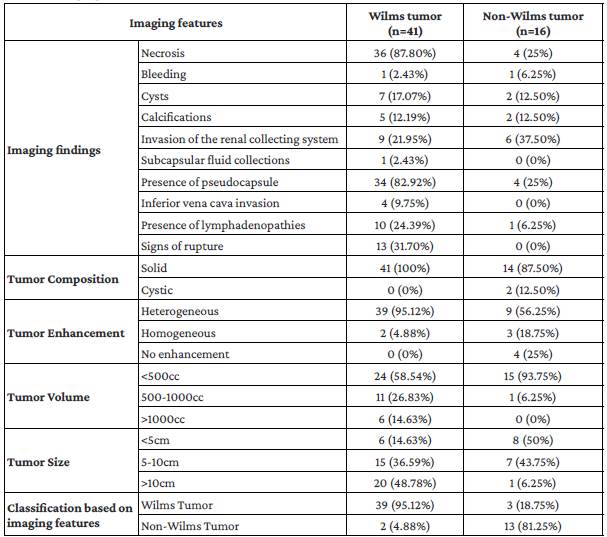
One child had a rhabdoid tumor in the right kidney; liver, lung and bone metastases were also detected at the time of diagnosis. CT scan images of this patient are shown in Figure 2.
Source: Picture Archiving and Communication System of the Radiology Service of the Fundación Hospital Pediátrico La Misericordia Illustration: Own elaboration.
Figure 2 1-year-old female with a non-Wilms tumor in the right kidney. Contrast-enhanced CT scan of the abdomen (a: coronal plane; b: axial plane) showing a solid mass of probable renal origin (right kidney) with curvilinear calcifications surrounding the tumor associated with pleural (white arrow in image c) and liver metastasis (d). According to the histopathology report, the mass was a rhabdoid tumor. The ilustration summarizes the extrarenal findings associated with the precense non-Wilms tumors.
Immature teratoma
One patient had an immature teratoma in the left kidney. The tumor was predominantly cystic and contained fat and calcium elements.
Angiomyolipoma
An angiomyolipoma was found in one patient (left kidney); the histopathology report described it as having poor fat content.
Neuroblastoma
A 5-month-old patient with an abdominal mass was assessed at the Radiology Service, with imaging findings suggestive of a kidney tumor. A perirenal lesion with extension to the renal pelvis on the right side was found intraoperatively. Histopathologically, the mass was diagnosed as an extra-adrenal perirenal neuroblastoma with kidney involvement.
Vascular malformations
A 14-year-old patient with a history of Klippel-Trenaunay syndrome was diagnosed with low-flow vascular malformations in both kidneys; the diagnosis was confirmed histologically after performing an emergency laparotomy due to a spontaneous hemoperitoneum.
Concordance analysis (agreement)
The kappa coefficient between radiological diagnosis of Wilms/non-Wilms tumors and histological diagnosis was 0.78 (95%CI: 0.59-0.96; p<0.05), that is, a moderate to very good concordance.
Tumor rupture
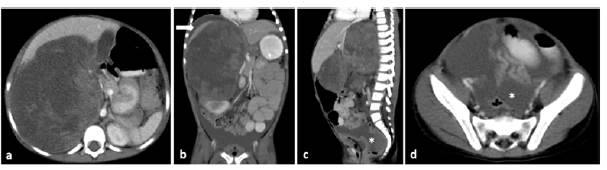
Signs of tumor rupture were found in the imaging studies of 13 Wilms tumors (presence of free fluid in the cul-de-sac, perirenal fat stranding, and presence of retroperitoneal free fluid). Such signs were not observed in the non-Wilms tumors group. Tumor rupture was reported intraoperatively in 6 tumors, all of them with imaging signs of rupture Figure 3.
Source: Picture Archiving and Communication System of the Radiology Service of the Fundación Hospital Pediátrico La Misericordia.
Figure 3 Imaging findings of tumor rupture in a 4-year-old female patient with a perioperative Wilms tumor rupture. Contrast-enhanced CT scan of the abdomen, axial (a and d), coronal (b) and sagittal planes (c), showing a solid mass with a tumor size >10cm and volume >1000cc in the right kidney. Imaging signs of rupture: fat stranding, presence of free perirenal fluid (white arrow in b) and of free fluid in the pelvic cavity and the cul-de-sac (asterisk in c and d).
The characteristics of the 41 Wilms-tumors, classified according to the surgical confirmation of tumor rupture, as well as the results of the univariate analysis carried out to assess the association between tumor variables (tumor size, volume and type) and peri-operative rupture are shown in Table 3. Tumor volume was associated with a higher risk of tumor rupture (OR: 3.08, 95%CI: 1.04-9.09; p<0.05).
Table 3 Association between tumor variables and peri-operative diagnosis of tumor rupture.
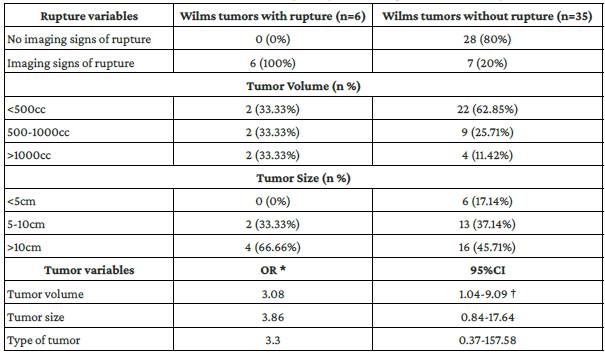
n: number, OR: odds ratio, CI: confidence interval.
*Association between tumor variables and peri-operative rupture (univariate analysis).
†Statistically significant association (p<0.05).
Source: Own elaboration based on the data obtained in the study.
Discussion
Although Wilms tumor was the most frequent renal tumor in the present study (71.93% of the tumors), this prevalence is lower to what has been described in the literature (at least 90% of renal tumor cases in pediatric population).1,2
Wu et at.,3 in a study conducted in China in 42 children (median age at diagnosis: 3 years 2 months) to compare specific CT scan findings between renal (n=16) and non-renal tumors (n=26) located in the perirenal space, reported that the presence of pseudocapsule, necrosis and cystic changes, vascularity, distant metastasis and inferior vena cava invasion were frequent findings in renal tumors (87.5%, 87.5%, 68.8%, 43.8%, and 31.3%, respectively). 3
In our study, the presence of tumor enhancement, pseudocapsule, and signs of rupture were significantly associated with having a histological diagnosis of Wilms tumor (OR: 21.6, 15.17, 14.57 and 8.21, respectively). Likewise, it was found that as volume and tumor size increase, there is a greater probability of diagnosing a Wilms tumor (OR: 7.93 and 4.37, respectively). Finally, an inverse correlation between the presence of metastasis and having Wilms tumors was observed (OR: 0.19).
On the other hand, in the non-Wilms tumors, there was a higher frequency of tumors with a volume <500cc than in the Wilms tumor group (93.75% vs. 58.54%); besides, the absence of tumor enhancement and the presence of invasion of the renal collecting system were also more frequent in this group (25% vs. 0% and 37.50% vs. 21.95%, respectively).
The usefulness of imaging findings to determine the type and grade of renal tumors in pediatric population is controversial since their staging can only be confirmed surgically and histopathologically. 16 In the United States, protocols of the National Wilms Tumor Study state that surgery should be performed before starting chemotherapy and that imaging studies should be used for preoperative planning. On the other hand, some European countries use the management protocol established by the International Society of Pediatric Oncology, which recommends using preoperative chemotherapy; in this scenario, the diagnostic approach is based on the clinical presentation and the imaging findings of the tumor, without the need of a biopsy. 16,22
Some studies have addressed the usefulness of computed tomography (CT) in the identification of tumor rupture by assessing imaging findings such as the presence of free fluid in the cul-de-sac, perirenal fat stranding and retroperitoneal fluid. For example, Khanna et at.,23 in a case-control study conducted in United States and in which 70 Wilms tumor cases with rupture were matched to 70 Wilms tumor controls without rupture according to age and tumor weight (<6 months and 50g), reported sensitivity and specificity values of CT in the detection of perioperative tumor rupture between 54%-70% (reviewer 1: 54% [36 of 67 cases; 95%CI: 41.1%-66.0%; reviewer 2: 70% [47 of 67 cases; 95%CI: 57.7%-80.7%] and 88% (reviewers 1 and 2: 88% [61 of 69 cases; 95%CI: 78.4%-94.9%).
In this regard, it should be noted that in the present study, the three abovementioned imaging signs of tumor rupture were observed in 13 of the 41 Wilms Tumors and that tumor rupture was confirmed in 6 of them. Furthermore, other studies recommend the administration of neoadjuvant chemotherapy only in patients <2 years old with tumors >1000g given the risk of rupture during surgery, as tumors <550g are considered to have good surgical prognosis. 24,25
Some of the limitations of this study include its retrospective nature and the small sample size due to the low incidence of these tumors. The results obtained here suggest that there is concordance between some imaging findings and histological diagnosis of Wilms tumor; however, the confidence intervals were wide, thus limiting the interpretation of said results. In addition, not all patients had ultrasound, CT and magnetic resonance imaging studies at the time of diagnosis, but it should be noted that assessing the accuracy of the different diagnostic modalities was not one of the objectives of this study.
Finally, it is worth noting that in the present study, on the one hand, the frequency of non-Wilms tumors (28.07%) was higher than what was expected according to what has been reported in the relevant literature, 1,2 and, on the other, the data collected allowed classifying renal tumors into Wilms or non-Wilms. Likewise, imaging signs that help determine the risk of tumor rupture, such as the presence of free fluid in the cul-de-sac, perirenal fat stranding and the presence of retroperitoneal free fluid, were also identified.
Conclusions
Based on the results of the present study, it is possible to conclude that there are imaging findings such as necrosis, tumor enhancement, presence of pseudocapsule and absence of metastasis that can help the radiologist predict the histological diagnosis of Wilms tumor.
On the other hand, even though it was not possible to establish specific imaging findings for the different types of tumors in the non-Wilms tumor group due to the small sample size, some characteristic findings were identified, including absence of rupture, involvement of other organs, volume <500cc, and absence of enhancement of the lesions.
In addition, a moderate to very good concordance between radiological diagnosis of Wilms/non-Wilms tumors and histological findings was found. Finally, tumor volume was associated with intraoperative finding of tumor rupture, information that could be useful in future works addressing the importance of this finding for surgical prognosis.