Introduction
Hereditary tubulopathies comprise a large group of diseases involving different segments of the nephron.1 Gitelman syndrome is one of these diseases and is rarely reported in the related literature.2 In Colombia, as of the date of this report, there are no studies or records that evaluate the prevalence of this syndrome in the general population, only isolated case reports.3 However, in 2017, Blanchard et al.4 established that worldwide prevalence is approximately 1 to 10 cases per 40 000 people, with a possible higher rate in Asia.
Gitelman syndrome is classified as an autosomal recessive hereditary tubulopathy caused by a mutation in the SCL12A3 gene located on the long arm of chromosome 16 (I6q13).5 There are approximately 500 known mutations in this gene, which codes for the thiazide-sensitive sodium/chlorine (Na-Cl) cotransporter located on the apical surface of the distal convoluted tubule. These mutations alter the normal function of this protein and therefore lead to sodium chloride reabsorption and volume depletion that ends up increasing the release of hormones such as renin and aldosterone, which in turn leads to potassium and hydrogen losses.5,6
This syndrome is an entity usually detected in adolescents or young adults4 and has a broad spectrum of manifestations ranging from mild symptoms, such as muscle weakness, fatigue, thirst, nocturia or cramps, to severe conditions, such as rhabdomyolysis, tetanus and even life-threatening arrhythmias.7 Similarly, Bettinelli et al.,8 in a study of 34 pediatric patients with primary renal tubular hypokalemic metabolic alkalosis divided into two groups, one of 16 participants with Bartter syndrome and one of 16 participants with Gitelman syndrome, found that the manifestations of the latter group were metabolic alkalosis hypokalemia with hypomagnesemia and hypocalciuria.
The following is the case of a patient with Gitelman syndrome, in which the clinical, paraclinical, and molecular diagnostic behavior of this disease is described in order to place it within the broad range of differential diagnoses of recurrent or refractory hypokalemia, as well as to present its therapeutic approach.
Case presentation
A 23-year-old mixed-race woman visited the emergency department of a quaternary care center in Cali, Colombia, due to intermittent muscle cramps in the lower limbs during the night, especially in the calves, lasting between 1 minute and 2 hours. These cramps, which had started three months earlier and were being treated with non-steroidal anti-inflammatory drugs (without complete remission), were accompanied by asthenia, adynamia, and a sensation of palpitations, and worsened shortly before the assessment due to gastrointestinal symptoms (emesis and fluid stools). Her only relevant clinical history included a hospital admission due to severe hypokalemia (1.9 mmol/L) one year earlier; on that occasion, she received intravenous fluids and no further studies were performed.
On admission, physical examination revealed hypotension (90/60 mmHg), signs of dehydration (xerostomia and tachycardia), intermittent muscle fasciculations in the lower limbs, and hyperreflexia. Laboratory tests showed severe hypokalemia with hypomagnesemia, normal renal function, hyperreninemia, and normal aldosterone levels (Table 1). Given the findings, potassium (central potassium chloride at 15 mEq/hour for 6 hours and then at 5 mEq/hour) and magnesium (1.5 g of intravenous magnesium sulfate every 8 hours) replacement therapy was initiated, which resulted in an improvement of her clinical condition after 3 days, when an electrolyte follow-up test showed normal ranges (K: 3.9 mEq/L and Mg: 1.10 mEq/L).
Table 1 Laboratory test results.
| Lab test | Result | Normal values | ||
|---|---|---|---|---|
| Potassium | 1.9 | 3.5-5.0 mEq/L | ||
| Magnesium | 0.7 | 0.85-1.10 mEq/L | ||
| Blood urea nitrogen (BUN) | 35 | 6- 20 mg/dL | ||
| Creatinine (Cr) | 0.9 | 0.6- 1.1 mg/dL | ||
| Arterial gases | pH | 7.51 | 7.35-7.45 | |
| Bicarbonate (HCO3) | 34 | 22-28 mEq/L | ||
| PCO2 | 38 | 35-45 mmHg | ||
| Plasma renin activity | 8.91 | 1.9-3.7 ng/mL/hour | ||
| Aldosterone | 16.8 | 0.0- 1.2 mg/dL | ||
| Aldosterone-to-renin ratio | 0.19 | <25 | ||
Source: Own elaboration.
Two days after completing the treatment, the patient had an electrolyte disorder again (K: 2.0 mEq/L and Mg: 0.7 mEq/L) and muscle cramps, this time without an apparent cause (absence of gastrointestinal symptoms).
The arterial blood gas test showed metabolic alkalosis. In addition, the urine electrolytes test (Table 2) found elevated levels of potassium, magnesium, chlorine, and sodium, and reduced levels of calcium. It was decided to restart potassium and magnesium replacement (peripheral at 4 mEq/L every 8 hours and 1.5 g intravenously every 8 hours, respectively) and to start additional administration of eplerenone at a dose of 25 mg/day. Normal electrolyte levels were observed in a follow-up test performed 3 days after treatment was restarted (K: 4.1 mEq/L and Mg: 1.2 mEq/L), thus the patient was prescribed a diet, oral potassium replacement with 15 cm3 of potassium gluconate every 8 hours, and an aldosterone antagonist administered at the same dose. Renal and urinary tract ultrasound was also performed, which allowed to rule out morphological alterations.
Table 2 Electrolytes in urine.
| Lab test | Result | Normal values |
|---|---|---|
| Potassium in spot urine sample | 193.8 | 20-80 mEq/L |
| Magnesium in spot urine sample | 38.3 | 2.4-7.4 mEq/L |
| Calcium in spot urine sample | 10 | 13.4-42 mEq/L |
| Sodium in spot urine sample | 168 | 20-110 mEq/L |
| Chlorine in spot urine sample | 222 | 55-125 mEq/L |
| 24-hour urine calcium | ≤1 | 2.5-7.5 mEq/24 hours |
Source: Own elaboration.
In a young patient with a history of recurrent hypokalemia of renal origin (elevated urine potassium) associated with hypomagnesemia, normocalcemia with hypocalciuria, contraction alkalosis, secondary hyperaldosteronism and hyperreninemia, and after ruling out other diagnostic possibilities, Gitelman syndrome was suspected. Consequently, genetic studies were requested to identify the causal mutation.
Amplification and sequencing analysis of the SCL12A3 gene revealed a homozygous intronic mutation within 7+1 G>T (NM_001126108.2(SLC12A3):c.964+1G>T), consisting of a substitution of guanine for thymine at position 964; since this is a donor splice site at intron 7, an absent or altered protein product is expected from this mutation.
Since the patient was stable and her symptoms disappeared, she was discharged with the following indefinite outpatient management: potassium gluconate oral solution 31.2% (15 cm3 every 8 hours), magnesium gluconate (550 mg orally every 8 hours), and eplerenone (50 mg/day orally). At her last follow-up, 1 year after recovery, her serum electrolyte levels were normal (K: 4.0 mEq/L and Mg: 1.26 meq/L) and she reported no recurrence of similar symptoms.
Discussion
Gitelman syndrome (entry #263800, according to the Online Mendelian Inheritance in Man® phenotype and genotype registry)9 is a rare renal tubular salt-wasting disorder that affects adolescents and young adults. It is caused by a dysfunction of the thiazide-sensitive Na/Cl cotransporter that results in a mutation of the SCL12A3 gene.5,10-13 This syndrome is characterized by biochemical abnormalities such as hypomagnesemia, hypocalciuria and secondary hyperaldosteronism, which induce hypokalemia and metabolic alkalosis, the latter two being recurrent when the appropriate treatment is not administered.10
Symptoms of Gitelman syndrome include cramps, paresthesia, fatigue, and recurrent episodes of emesis, diarrhea, or fever accompanied by carpopedal spasms and hypotension.11 In this sense, its diagnosis is based on the clinical symptoms associated with biochemical abnormalities and is confirmed by molecular biology techniques.10
The patient presented in this case report had a history of hospitalization for hypoka-lemia for which potassium replacement therapy was prescribed. She consulted due to intermittent cramps in her lower limbs over the past three months, which became severe due to gastrointestinal symptoms, as described in the medical literature.10-13
Laboratory tests performed on admission detected hypokalemia once again; since this is a condition associated with biochemical alterations characteristic of Gitelman syndrome,10,11 systemic diseases such as congestive heart failure or cirrhosis were ruled out.12 As a result, two genetic disorders were considered as differential diagnoses: Gitelman syndrome and Bartter syndrome.3,5 The latter was considered improbable since it usually manifests at a younger age and with a more severe phenotype, and because urine calcium excretion is often increased and magnesemia is normal or slightly reduced11,13 (Table 3).
Table 3 Differences between Bartter and Gitelman syndromes.
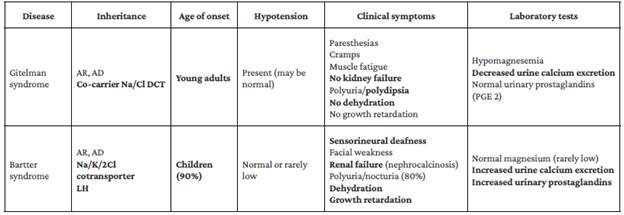
AR: autosomal recessive; AD: autosomal dominant; DCT: distal convoluted tubule; LH: loop of Henle. Note: Major differences are highlighted in bold.
Source: Own elaboration based.
According to the literature, the patient's symptoms are explained by the loss of sodium chloride caused by dysfunction of the thiazide-sensitive Na/Cl cotransporter secondary to a mutation of the SCL12A3 gene. This dysfunction is accompanied by decreased magnesium absorption and increased calcium reabsorption in the first part of the distal convoluted tubule, resulting in hyperreninemia and secondary hyperaldoste-ronism due to an increase in urinary sodium concentration, as well as increased sodium and water reabsorption by the principal cells and increased potassium secretion in the nephron. Likewise, the intercalated cells located in the collecting ducts secrete a greater amount of hydrogen ions due to a predominantly negative intratubular charge (by chlorine ions).
As mentioned above, the diagnosis of Gitelman syndrome is confirmed by DNA mutation analysis of the SCL12A3 gene, in which more than 500 mutations have been detected.6 The mutation detected in the patient of the case described here (homozygous intronic mutation in 7+1 G>T [NM_001126108.2(SLC12A3):c.964+1G>T], consisting of a substitution of guanine by thymine at position 964, is very rare, with few descriptions in the literature.12,14
The recommended treatment for Gitelman syndrome is magnesium supplementation combined with a diet high in sodium and potassium, as well as potassium replacement (40-100 mEq potassium chloride/day). If there is no improvement in potassium levels, it is necessary to administer potassium-sparing drugs such as aldosterone antagonists (up to 100mg of spironolactone orally per day or 25mg of eplerenone orally every 12 hours) or sodium channel blockers (5mg of amiloride orally every 12 hours).15 In accordance with this, the patient reported here received management with diet, potassium replacement and eplerenone, which led to a favorable course.
Figure 1 presents the algorithm used to reach the patient's definitive diagnosis. According to this algorithm, the young woman presented hypokalemia (Figure 1a), increased urinary potassium values (>40 mmol/L) (Figure 1b), low blood pressure (Figure 1c), and metabolic alkalosis (Figure 1d). In contrast, she did not present comorbidities such as heart failure or cirrhosis (without clinical manifestations) (Figure 1e). Based on the findings, two tubulopathies were suspected: Bartter syndrome and Gitelman syndrome; however, the former was ruled out because the symptoms were more associated with the latter. In this regard, it was decided to request a genetic analysis that documented mutations in the SCL12A3 gene and confirmed the diagnostic suspicion. All of the above confirmed that the sequential approach to a young patient with hypokalemia is useful to establish a timely diagnosis and provide adequate treatment.
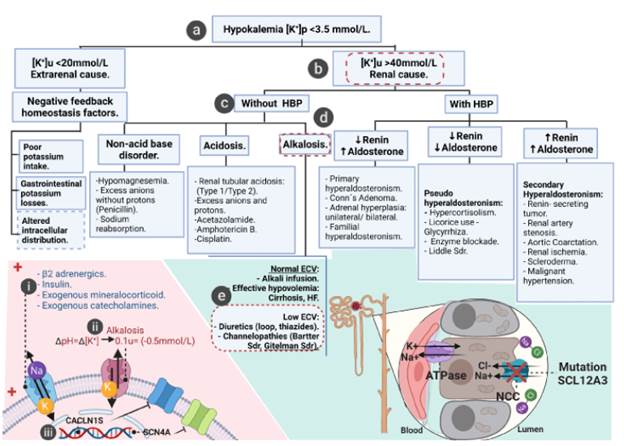
K: potassium; HBP: high blood pressure; ECV: extracellular volume; HF: heart failure. Source: Elaboration based on Vidal-Petiot et al.12 The image was created using BioRender.com
Figure 1 Hypokalemia diagnostic algorithm.
Conclusion
Gitelman syndrome is a rare disease with nonspecific symptoms, so an adequate sequential approach to a patient with recurrent hypokalemia is of great importance for making an accurate diagnosis. Likewise, it allows for effective and timely management that favors long-term prognosis and reduces hospital readmission rates.















