Introduction
Pneumonia is an infection that can affect one or both lungs and presents with symptoms ranging from mild to severe, including cough (with or without mucus), fever, chills, and shortness of breath. The degree of severity of pneumonia depends on factors such as age, general health condition, and the origin of the infection.1 Although pneumonia can occur in people of all ages, the age groups most at risk for developing pneumonia and of it becoming severe are children aged 2 years and younger, and persons over 65 years of age.1 In fact, it has been reported that, compared to other age groups, the highest overall pneumonia mortality rate is reported in people aged 70 years or older, with 261 pneumonia deaths per 100 000 people in 2017 for this age group.2 Moreover, also in 2017, the overall mortality rate for pneumonia was 15 deaths per 100 000 people.2
In the United States, pneumonia was the most common primary diagnosis in hospitalized patients in 2014 (41.2% in intensive care unit [ICU] patients and 36.6% in inpatients), and respiratory diseases were the most common cause of death in patients with two or more prior emergency department visits (36.1%) and the second most common cause in those with one prior emergency department visit (25.9%).3 In Colombia, in 2010, the population aged 80 years or older was the age group with the highest annual consultation and hospitalization rates (873 consultations and 100 hospitalizations per 1 000 person-years, respectively), while lower respiratory tract infection was the disease with the second highest annual incidence rate (2 232 new cases per 100 000 persons).4
Besides its impact in terms of morbidity and mortality, and because the use of antimicrobials is one of the therapeutic options to be considered in pneumonia, its proper diagnosis and treatment has an impact on the development of antimicrobial resistance. Since 2014, this situation has been considered a public health problem by the World Health Organization (WHO),5 which included community-acquired microorganisms such as Streptococcus pneumoniae, one of the main etiological agents of community-acquired pneumonia (CAP), in the list of species of greatest concern for antimicrobial resistance.6 CAP is defined as acute parenchymal lung infection that is acquired in a community setting and is not associated with health care in a hospital, nursing home, or other health care setting.7
Therefore, establishing guidelines to direct the treatment of this group of patients is important in order to obtain better clinical outcomes and promote a rational use of antibiotics in this setting. In Colombia, the last national guideline for the management of CAP was published in 2013,8 so it is necessary to review and update the recommendations for the management of this infection in the country, without overlooking the specific recommendations for the care of COVID-19 patients,9 which, depending on the need or appearance of new evidence, may be reviewed and updated eventually.
Scope of the clinical practice guideline (CPG)
This CPG is intended for health care workers involved in the care of adult patients (>18 years of age) with a clinical suspicion or confirmed diagnosis of CAP and for decision makers or entities involved in the generation of health policies related to the management of this condition. This CPG includes recommendations for the diagnosis and treatment of CAP in the Colombian context.
Objective of the clinical practice guideline
To systematically generate evidence-based clinical recommendations for the diagnosis and treatment of adult patients with a clinical suspicion or confirmed diagnosis of CAP in order to optimize the quality of health care provided to these patients in the country and, thus, obtain better clinical outcomes and promote the appropriate and safe use of antimicrobials in the treatment of this population.
Population targeted by the CPG
The recommendations contained in this CPG address the following patient groups:
Population not targeted by the CPG
The recommendations contained in this CPG do not include the following population groups:
Pediatric population (<18 years of age).
Pregnant women
Patients with clinical suspicion or confirmed diagnosis of health care-associated pneumonia.
Patients with clinical suspicion or confirmed diagnosis of ventilator-associated pneumonia.
Patients with acute exacerbation of chronic obstructive pulmonary disease.
Patients with primary or secondary immunodeficiency.
Intended users of the CPG
The recommendations contained in this CPG are aimed at health workers involved in the health care process of adult patients with a clinical suspicion or confirmed diagnosis of CAP at the different levels of health care of the Colombian General Social Security Health System (I, II, III, and IV), namely, general practitioners; specialists in emergency medicine, family medicine, internal medicine, critical medicine and intensive care, pulmonology, and infectious diseases; nurse practitioners; clinical laboratory staff; pharmaceutical chemists; and other personnel involved in the diagnosis and treatment of patients with CAP.
Methodology
This CPG was developed in accordance with the standards established in the Guía Metodológica Adopción - Adaptación de Guías de Práctica Clínica Basadas en Evidencia (Methodological Guidelines for the Implementation-Adaptation of Evidence-Based Clinical Practice Guidelines) of the Ministry of Health and Social Protection of Colombia,10 as described below.
Composition of the guideline development group
The guideline development group (GDG) consisted of eight members distributed as follows: two specialists in adult infectious diseases (JAC, SIC), four specialists in adult pulmonology (EAS, AS, JVT, DPA), one specialist in internal medicine (LCN), and two clinical epidemiologists with experience in the development of CPGs, systematic literature reviews, synthesis and qualification of evidence, and participatory processes (JAC, MCV).
Prior to the start of the development of the GPC, each of the GDG members declared whether or not they had conflicts of interest by filling out a conflict-of-interest disclosure form designed for this purpose. When a conflict of interest was declared, an analysis was carried out to define its impact on the member's participation in the different CPG development activities.
The scope and objectives of the CPG, as well as the aspects to be addressed, were defined during informal consensus meetings in which all members of the GDG participated.
GPC search
Once the scope and objectives of the CPG were established, a systematic search of CPGs was conducted to identify guidelines published in any language between 2015 and 2020 that addressed the issues set out in the scope of the present guideline.
The websites of the following CPG compilers and developers were consulted: Guidelines International Network, Agency for Healthcare Research and Quality/National Guidelines Clearinghouse, CMA Infobase: Clinical Practice Guidelines, Catálogo de Guías de Práctica Clínica en el Sistema Nacional de Salud (Guía Salud), National Institute for Clinical Excellence (NICE), Scottish Intercollegiate Guidelines Network, New Zealand Guidelines Group, and American College of Physicians.
Systematic searches were also performed in MedLine, LILACS and Epistemonikos using search strategies adapted for each search engine and utilizing Boolean, truncation and proximity operators, as well as free-text terms and controlled vocabulary, including key terms such as "community-acquired infections" and "respiratory tract infections". The Medline search strategy is outlined in Supplementary Table 1.
Screening, quality assessment, and selection of CPGs to be used for the development of the guideline
Once the searches were performed, and after removing duplicates, two reviewers (MCV, LCN) independently performed the primary screening of the records retrieved by reading the title and abstract, selecting the documents classified as CPG or evidence-based recommendations that addressed at least one of the aspects defined in the scope of this guideline. Subsequently, two reviewers (MCV, LCN) independently performed the secondary screening (full-text reading) of the references selected in the previous step, using the modified 7 tool (i.e., Guideline Implementability Appraisal tool) proposed in the Guía Metodológica Adopción - Adaptación de Guías de Práctica Clínica Basadas en Evidencia of the Colombian Ministry of Health and Social Protection.10 Disagreements on the inclusion of a CPG were resolved by consensus or through the intervention of a third reviewer in cases where agreement between the two reviewers could not be reached.
Even though, as mentioned above, there were no publication language restrictions in the CPG searches, studies published in languages such as German, Japanese or Korean were excluded during the full-text reading screening stage and only those published in English or Spanish were taken into account, because the reviewers were not fluent in the former languages.
Next, the selected CPGs were presented to the development group in order to evaluate their methodological quality using the AGREE II tool.11 Each guideline was evaluated independently by three reviewers, and at least one clinical expert and one methodological expert always participated in the process. Furthermore, when there was doubt as to the information available for the evaluation of the CPG, additional information from the developer groups was requested by email.
After completing the quality assessment process, CPGs with a score >60% in the domains of methodological rigor and editorial independence were selected. Finally, in accordance with the recommendations of the Guía Metodológica Adopción - Adaptación de Guías de Práctica Clínica Basadas en Evidencia of the Colombian Ministry of Health and Social Protection,10 and with the aim of selecting the CPGs to be used in the adaptation process, the GDG analyzed the selected guidelines taking into account the following aspects: the topics addressed in the guidelines, the use of the GRADE methodology, the year of publication, and the date of the last search for evidence reported in the guideline.
Based on the methodology described above, two CPGs were selected for the adaptation process, namely, the American Thoracic Society and Infectious Diseases Society of America (ATS / IDSA) CPG: Diagnosis and Treatment of Adults with Community Acquired Pneumonia, an Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America,12 and the National Institute for Clinical Excellence (NICE) CPG: Pneumonia (community-acquired): antimicrobial prescribing [NG138],13 both published in 2019. To adapt the recommendations to the Colombian context, authorization was obtained from the authors of both CPGs (Metlay JP and NICE).
Adaptation of recommendations
The GDG carried out the adaptation process using the GRADE methodology, (specifically the GRADE EtD tool),14,15 based on the information provided in the two selected CPGs. The GRADE evidence profiles were also included along with the evaluation of the certainty of the evidence, which was completed by analyzing each of the following domains: number and design of studies, risk of bias, inconsistency, indirect evidence, imprecision, and other considerations. All this was done taking into account considerations specific to the Colombian context, the benefits and harms of the different options, the use of resources, the impact on equity, and the feasibility of implementation.
Expert consensus
The adapted recommendations were presented at three expert consensus meetings with the participation of representatives from different health sciences disciplines and scientific societies ("Asociación Colombiana de Infectología - ACIN [JP, FOG, IR], Asociación Colombiana de Neumología y Cirugía del Tórax - Asoneumocito [FM], Asociación Colombiana de Medicina Interna - ACMI, Asociación Colombiana de Medicina Crítica y Cuidado Intensivo - AMCI [JARC], professionals in the areas of microbiology, respiratory therapy, pharmacy, nursing and health auditing [ALL, IJG, SLC, JAD, NG, FG]), as well as patient representatives (Asociación de usuarios de Méderi [JEM, MP]), thus including the perspective of the various stakeholders.
At the consensus meeting, through a participatory process using Delphi methodology in real time and taking into account the information and evidence provided by the two selected CPGs and the considerations specific to the Colombian context suggested by the clinical experts, recommendations were formulated and graded according to the GRADE16 methodology, and good practice points were formulated, which allow good clinical practice in patient management.
Voting during consensus was conducted anonymously and electronically. Agreement was defined as reached if more than 50% of the voters were in favor or against a recommendation or good practice point. In cases in which no agreement was reached in the first round, a discussion session was held followed by a new voting round; a maximum of three voting rounds were allowed for each recommendation and/or good practice point.
Quality of evidence grades
High: We are very confident that the true effect is close to the estimate of effect.
Moderate: We are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it could be substantially different.
Low: Our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect.
Very low: We have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect.
Implications of strong and weak recommendations
Implications of a strong recommendation are:
For patients: Most individuals in this situation would want the recommended course of action and only a small number would not.
For clinicians: Most individuals should receive the recommended course of action.
For policy makers: The recommendation can be adapted as policy in most situations.
Implications of a weak (conditional) recommendation (suggestion) are:
For patients: The majority of individuals in this situation would want the suggested course of action, but many would not.
For clinicians: Recognize that different choices will be appropriate for different patients, and that you must help each patient arrive at a management decision consistent with her or his values and preferences.
For policy makers: Policy making will require substantial debates and involvement of many stakeholders.
Questions addressed in the guidelines
Should sputum Gram stain and culture be performed for diagnosing CAP in adults?
Should blood cultures be performed for diagnosing CAP in adults?
Should urinary antigen testing be performed for diagnosing CAP in adults?
Should influenza virus testing be used for diagnosing CAP in adults?
Should clinical prediction rules be used to determine the healthcare setting in which adult CAP patients should be treated?
Should the procalcitonin test be used to define the initiation of antimicrobial therapy in adults with CAP?
What is the best strategy for empirical antimicrobial therapy in adult outpatients with CAP?
What is the best strategy for empirical antimicrobial treatment in adult inpatients with CAP?
What is the best strategy for empirical antimicrobial treatment in adult inpatients with CAP and risk factors for methicillin-resistant Staphylococcus aureus (MRSA) or Pseudomonas aeruginosa?
What is the appropriate duration of antimicrobial therapy in adult patients with CAP, both outpatients and inpatients?
What is the appropriate route of administration of antimicrobial therapy in adult patients with moderate to severe CAP?
Should antiviral therapy be included in the treatment of adult patients with CAP who test positive for influenza?
Should corticosteroids be used in adult inpatients with CAP?
Recommendations and evidence review
Recommendations
We do not recommend routine sputum Gram stain and culture in adult outpatients with CAP (strength of recommendation: strong against; quality of evidence: very low ⨁◯◯◯).
We recommend performing Gram stain and culture of lower respiratory tract secretions prior to initiating antimicrobial therapy in hospitalized adult patients with CAP who:
° Have been diagnosed with severe CAP (Table 1) (strength of recommendation: strong for; quality of evidence: very low ⨁◯◯◯); or
° Are receiving empiric antimicrobial therapy for MRSA or P. aeruginosa (strength of recommendation: strong for; quality of evidence: very low ⨁◯◯◯); or
° Have a history of MRSA or P. aeruginosa infection, especially patients with previous respiratory tract infection with one of these pathogens (strength of recommendation: weak for; quality of evidence: very low ⨁◯◯◯); or
° Have a history of hospitalization and antimicrobial therapy in the last 90 days (strength of recommendation: weak for; quality of evidence: very low ⨁◯◯◯).
Table 1 Criteria for severity of community-acquired pneumonia.
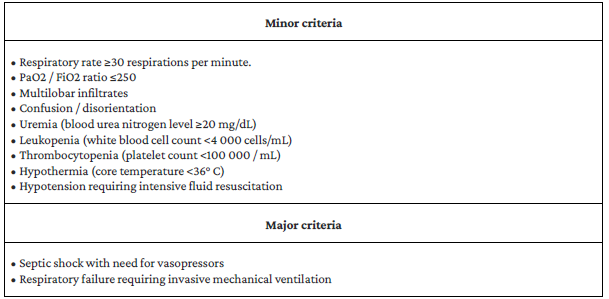
CAP is considered severe if the patient meets 1 major or 3 or more minor criteria.
Source: Adapted from Mandell et.al. 17
Good clinical practice point
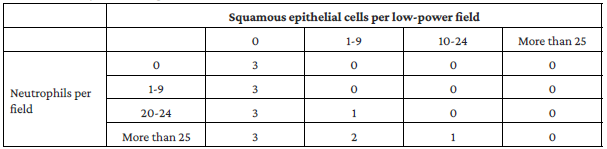
Respiratory tract specimen quality should be judged based on the Murray & Washington criteria,18 and the report should be interpreted only if the specimen result is category 2 or 3. See Table 2.
Rationale for the recommendation
Gram staining is an easy and quick test that can be performed in most health institutions, even in low complexity centers. Initially designed to identify S. pneumoniae, it allows to rapidly determine the microbial etiology of CAP. Conversely, sputum culture entails more difficulty due to the usual limitations for the growth of S. pneumoniae and a number of important causative microorganisms, especially intracellular bacteria.
In clinical practice, according to what has been reported in the literature, the results of sputum Gram stain or culture may have a very limited impact on decisions regarding the treatment of CAP in the outpatient setting. Given the low quality of evidence of the benefit of performing cultures of respiratory specimens from adult patients with CAP in the outpatient setting, the consensus group agreed to recommend against performing sputum Gram stain and culture in this context.
However, there are two cases in which sputum culture and Gram stain are recommended. The first is endotracheal aspirate culture in patients with severe CAP requiring intensive care with endotracheal intubation, since McCauley et al. 19 found that, for intubated patients with CAP, tracheal aspirate culture was the only positive test in 39% of cases (32 of 89) when other diagnostic tests such as blood cultures and urinary antigen tests were negative. The second case in which we recommend performing sputum culture and Gram stain is patients with suspected MRSA or P. aeruginosa infection to confirm their etiology and/or adjust antibiotic treatment based on the results of these tests; although evidence on risk factors associated with the presence of these microorganisms is not solid, it has been reported that situations such as a history of infection by these bacteria and previous antibiotic treatment or hospitalization in the last 90 days may be associated with an increased risk of MRSA or P. aeruginosa.
In addition, sputum cultures on admission in referral hospitals may be helpful in decision-making regarding antimicrobial therapy in these patients, as well as in identifying those who have been admitted with previous airway colonization. This recommendation aims to promote the rational use of antimicrobials when treating these patients by enabling the adjustment of the antimicrobial scheme once the culture results are available.
Summary of evidence
The studies that were identified to establish these recommendations are observational and, in general, the quality of the evidence is very low. Firstly, the criteria for differentiating patients with severe CAP are set out in Table 1,17 while the Murray and Washington criteria for interpreting the sputum Gram stain are presented in Table 2.18 Signori et al, 20 in a study involving 274 hospitalized patients with CAP, evaluated the frequency of sputum culture and its association with mortality, finding a mortality rate of 32.7% in patients who did not present with expectoration (n=58), 20.9% in patients with expectoration but from whom sputum samples were not collected for culture (n=124), and 6.5% in patients from whom sputum samples were collected for culture (n=124), with a higher risk of mortality in the group with expectoration but from whom sputum samples were not collected (OR: 3.78, 95%CI: 1.40-10.23). Moreover, in the study by Uematsu et al., 21 conducted in Japan with data from 65 141 adult hospitalized patients with CAP, no association was found between sputum testing and 30-day mortality (OR: 1.06, 95%CI: 0.98-1.15; p=0.013), nor with length of hospital stay (HR: 0.98, 95%CI: 0.97-1.00; p=0.071).
Studies on the usefulness of sputum Gram staining in the hospital setting, such as the one conducted in Japan by Sato et al. 22 in 144 CAP cases requiring hospitalization, have shown no significant difference in length of hospital stay (9.67 days with the test vs. 11.75 days without the test, p=0.053) nor in time on intravenous antimicrobial therapy (6.73 days with sputum Gram staining vs. 7.91 days without the test, p=0.44). These findings have been confirmed in other studies on microbiological testing that have shown that establishing an etiologic diagnosis using sputum Gram stain or culture routinely in settings with a low frequency of resistant pathogens does not have a significant impact on mortality, length of hospital stay, changes in antimicrobial therapy, or the overall clinical prognosis of these patients.23,24
In Colombia, there is insufficient information to evaluate the economic impact of the implementation of these recommendations. In the existing literature, no local data on the costs of medical care for CAP were found. Although the consensus meeting took into account data on the costs of some supplies, medications, hospitalization, among other factors involved in the care of these patients in the country, it was not possible to make an actual assessment of the cost-effectiveness of these recommendations. There were also no studies that allowed us to evaluate, from any perspective, the effect of the recommendations on health equity in minority or disadvantaged populations. These two aspects were considered as issues to be investigated in Colombia in order to better establish potential recommendations in future versions of the guideline.
Question N° 2. Should blood cultures be performed for diagnosing CAP in adults?
Recommendations
We do not recommend performing blood cultures in adult outpatients with CAP (strength of recommendation: strong against; quality of evidence: good: ⨁◯◯◯).
We recommend performing blood cultures prior to initiating antimicrobial therapy in hospitalized adult patients with CAP who:
Have been diagnosed with severe CAP (Table 1) (strength of recommendation: strong for; quality of evidence: very low ⨁◯◯◯); or
Have risk factors for MRSA or P. aeruginosa (strength of recommendation: strong for; quality of evidence: very low ⨁◯◯◯); or
Have a history of MRSA or P. aeruginosa infection, especially patients with a history of respiratory tract infection by one of these pathogens (strength of recommendation: weak for; quality of evidence: very low ⨁◯◯◯); or
Have a history of hospitalization and antimicrobial therapy in the last 90 days (strength of recommendation: weak for; quality of evidence: very low ⨁◯◯◯).
Good clinical practice point
The blood culture set should be collected according to the parameters defined in the manual of procedures for sample collection issued by the Instituto Nacional de Salud (National Health Institute or INS by its acronym in Spanish).25
Rationale for the recommendation
Blood cultures allow the identification of microorganisms in the blood at the time of CAP diagnosis. However, most patients will not present with bacteremia, which is limited to S. pneumoniae, S. aureus, and Enterobacteriaceae infections, and to patients with comorbidities or severe forms of the disease.
Evidence on the benefit of performing blood cultures in all CAP patients has a very low quality, so the recommendation is limited to certain groups of patients who could be considered at higher risk, such as those with severe CAP or with risk factors for microorganisms such as MRSA or P. aeruginosa. In addition, routine performance of this test may lead to false positives that promote indiscriminate use of antimicrobials in the context of CAP treatment. Another reason for not recommending the routine performance of blood cultures is that results may take more than 48 hours, so treatment adjustment may be delayed or no longer relevant in outpatients or inpatients with mild or moderate CAP.
Summary of evidence
The reviewed studies on the usefulness and benefit of taking blood cultures in CAP patients are observational. For instance, Meehan et al., 26 in a study evaluating which care strategies in hospitalized CAP patients were associated with mortality outcome in a sample of 14 069 older adults (>65 years) hospitalized in different parts of the United States, found that the performance of blood cultures, both before initiating antibiotic administration and within the first 24 hours of administration, did not show a benefit with respect to 30-day mortality (adjusted OR: 0.92, 95%CI: 0.82-1.02; p=0.10 and adjusted OR: 0.90, 95%CI: 0.81-1.00; p=0.07).
Similarly, another study conducted in the United States in 1 062 adult inpatients with CAP reported that the performance of blood cultures within the first 24 hours after hospital admission or before the administration of antimicrobials had no impact on mortality (adjusted OR: 0.86, 95%CI: 0.36-2.07 and adjusted OR: 1.21, 95%CI: 0.62-2.34, respectively), nor on length of hospital stay (adjusted OR: 1.04, 95%CI: 0.72-1.50 and adjusted OR: 0.84, 95%CI: 0.60-1.17, respectively). However, there was an association between performing blood cultures during the first 24 hours after hospital admission and an increased risk of clinical instability at 48 hours (adjusted OR: 1.62, 95%CI: 1.13-2.33).27
Along these lines, Costantini et al., 28 in a study comparing two cohorts of CAP patients admitted to a hospital in Italy in 2005 (n=234) and 2012 (n=321), found that the performance of this test was not associated with a benefit for in-hospital mortality (OR: 0.67, 95%CI: 0.37-1.21), nor for 30-day mortality (OR: 0.6, 95%CI: 0.32-1.09). Finally, it has been described that, in the context of routine blood culture performance in patients with non-severe CAP, the rate of contaminated blood cultures is 3.1% and that, consequently, it is likely that there are many more false positives (e.g., growth of coagulase-negative staphylococci that colonize the skin of patients and are not related to CAP) than true positives, thus promoting the indiscriminate use of antibiotics in the treatment of these patients.29
Question No. 3. Should urinary antigen testing be performed for diagnosing CAP in adults?
Recommendations
We suggest performing pneumococcal urinary antigen testing in adults with severe CAP (Table 1) (strength of recommendation: weak for; quality of evidence: low ⨁⨁◯◯).
Rationale for the recommendation
Randomized clinical trials of urinary antigen testing for Legionella and S. pneumoniae have not shown a clear benefit in terms of clinical outcomes in patients with severe CAP requiring ICU management. On the other hand, observational studies unfortunately have a low quality, and their results are often contradictory: some suggest that urinary antigen testing for Legionella is associated with a decrease in mortality in hospitalized patients with CAP, while others conclude that there is no such benefit.
Furthermore, no studies evaluating only the role of urinary antigen testing in hospitalized patients with severe CAP were found; instead, the studies include other diagnostic laboratory tests and multiple care strategies, so it is unclear whether the results can be attributed to urinary antigen testing alone or to diagnostic and therapeutic strategies in general. Although Legionella infection carries a high burden in terms of mortality in the context of severe CAP, we consider that the benefit of performing urinary antigen testing to determine its presence is very limited considering that this microorganism would already be covered by the empirical antimicrobial treatment scheme implemented in CAP patients considered to be at high risk. Regarding the performance of pneumococcal urinary antigen testing, the consensus group agreed to recommend this practice in patients with severe CAP, since it has been reported that its use may be related to a decrease in mortality.28
Summary of evidence
Evidence on the use of urinary antigen testing for detecting microorganisms when diagnosing CAP is conflicting. For example, Costantini et al 28 found that taking these tests, including pneumococcal and Legionella urinary antigen tests, was associated with a decrease in in-hospital mortality and 30-day mortality (OR: 0.427, 95%CI: 0.215-0.850 and OR: 0.341, 95%CI: 0.170-0.685, respectively), a finding also reported by Uematsu et al. 21 for 30-day mortality (OR: 0.75, 95%CI: 0.69-0.82; p<0.001).
On the other hand, Falgera et al.,30 in a study of 177 hospitalized CAP patients evaluating the impact of a treatment strategy based on the urinary antigen test result (n=88) compared to empirical treatment (n=89), showed, in a intention-to-treat comparative analysis, that the performance of this test did not have any benefit in terms of mortality, clinical relapse, ICU admission, days of hospital stay, need for readmission, adverse events, duration (in days) of antimicrobial treatment, and duration (in days) of intravenous treatment.
Question N° 4. Should influenza virus testing be used for diagnosing CAP in adults?
Recommendations
We recommend testing for influenza viruses, preferably using rapid molecular assays for influenza viral RNA or nucleic acid detection, rather than the rapid influenza antigen detection test in patients:
Diagnosed with severe CAP (Table 1) (strength of recommendation: strong for; quality of evidence: moderate ⨁⨁⨁◯), or
Who have a comorbidity (respiratory, cardiovascular or immunosuppression) (strength of recommendation: strong for; quality of evidence: moderate ⨁⨁⨁◯).
Rationale for the recommendation
Evidence on the usefulness of testing for influenza viruses in patients with CAP is scarce; moreover, the reported results are controversial. Available information comes from studies conducted in patients with lower respiratory tract infection, in a group of individuals with CAP, and not in a population of patients infected only with influenza. Epidemiological studies have reported that the prognosis of patients with severe CAP or CAP and comorbidities worsens when they have an infection caused by one of the influenza viruses, so the consensus group decided to recommend molecular assays for influenza viral RNA or nucleic acid detection in these two clinical scenarios.
Summary of evidence
No studies evaluating the usefulness of testing for influenza viruses in patients with CAP were found. The few papers found involved individuals with lower respiratory tract infection, such as the randomized clinical trial conducted by Oosterheert et al.31 in 107 patients (51.4% with CAP) to evaluate the feasibility and clinical and economic impact of using the TaqMan PCR test for the detection of respiratory viruses and atypical pathogens, in which it was found that, compared to the control group (n=52; use of conventional diagnostic procedures) there was no change in 28-day mortality, duration of antimicrobial treatment, or length of hospital stay.
Question N° 5. Should clinical prediction rules be used to determine the healthcare setting in which adult CAP patients should be treated?
Recommendations
We recommend using clinical judgment along with a validated clinical prediction rule, either the pneumonia severity index (PSI) or the CURB-65 or CRB-65 pneumonia severity scores, to assess the severity of illness and determine the need for hospitalization in adults diagnosed with CAP (strength of recommendation: strong for; quality of evidence: low ⨁⨁◯◯).
Good clinical practice point
Clinical judgment, along with the 2007 IDSA/ATS pneumonia severity criteria, should be used to determine the level of inpatient care in adults diagnosed with CAP who do not require vasopressors or mechanical ventilation.
Adults with CAP and hypotension requiring vasopressor therapy or with respiratory failure requiring mechanical ventilation should be admitted directly to the ICU.
Rationale for the recommendation
The use of clinical prediction rules to determine where patients with CAP should be treated (outpatient setting, general ward, or ICU) has been shown to be more effective and safer than clinical judgment, reducing variability in this decision among health care professionals. For patients with CAP classified for outpatient treatment and follow-up, a decrease in the cost of care and in the development of complications associated with inpatient treatment has been reported.
Even though the use of the severity and clinical prediction scores may vary because, depending on the level of care, laboratory and diagnostic tests required to calculate the values of these scores are not available in all health care centers, we recommend using one of these scores, with no preference in particular, to determine the health care setting in which these patients should be treated. In any case, besides the use of clinical prediction rules, the decision must be made taking into account clinical judgment, which includes the variability of the aspects considered in the prediction rules as well as socio-demographic, economic and support network factors of the patient.
Summary of evidence
Clinical prediction rules such as the PSI and CURB-65 pneumonia severity scoring system use demographic variables and clinical data obtained from laboratory and diagnostic tests to determine the prognosis of patients with CAP, especially 30-day mortality outcome.32-34 In this regard, Marrie et al.,35 in a multicenter controlled clinical trial conducted in 1 743 patients with CAP treated at the emergency department aimed to determine whether a critical care strategy that included clinical prediction rules had benefits in the treatment of these patients, found that using the PSI was a superior strategy in terms of classifying patients requiring outpatient treatment compared to not using any clinical prediction rules. They also found that the use of this score showed a benefit in terms of length of hospital stay, with a reduction in hospital readmission. These findings have been confirmed in other studies that have demonstrated that the use of the PSI in this population is safe, especially for selecting patients who only require outpatient care, and that this score has greater discriminating power to define the initial place of treatment for patients with CAP, without increasing the risk of mortality or morbidity.36,37
Studies on other clinical prediction rules for pneumonia are scant and results are less consistent. For example, in the case of the CURB-65 score, a randomized trial conducted in New Zealand comparing a group of patients with CAP who received inpatient treatment on a general ward (n=25) with a group who received outpatient treatment (n=24) had limitations in determining the differences between the two treatment strategies. One of them was related to the intravenous administration of antimicrobials under the supervision of nursing staff in the outpatient setting, which is a practice that is typical of the hospital setting and is referred to as "hospital-at-home" in several regions.38
The CURB-65 score includes simple variables that can be routinely recorded in the medical records, without the need for additional laboratory tests other than the blood urea nitrogen test, which facilitates its use in less complex health care settings and makes it an easier tool to use compared to other clinical prediction rules.39 In this regard, Aujeski et al., 40 in a study performed in 3 181 patients with CAP seen in the emergency departments of 32 hospitals of the United States and in which the performance of the PSI, CURB-65 and CURB clinical prediction rules for predicting 30-day mortality was evaluated, found that the diagnostic performance of the PSI was superior with an ROC curve of 0. 81 (95%CI: 0.78-0.84), showing a significant difference (p<0.01) in comparison with the other two scores (CURB: AUC=0.73, 95%CI: 0.68-0.76; CURB-65: AUC=0.76, 95%CI: 0.73-0.80).40
Question N° 6. Should the procalcitonin test be used to define the initiation of antimicrobial therapy in adults with CAP?
Rationale for the recommendation
Some of the studies that have described the use of the PCT test to determine the initiation of antimicrobial therapy in patients with CAP do not clearly meet the diagnostic criteria for pneumonia, and some do not even include the performance of an imaging study to confirm the diagnosis. For this reason, results about the benefit of performing this test in these patients may not be sufficiently reliable and conclusions may be controversial. For the GDG, the latter factor has the greatest weight for not recommending the PCT test to determine the initiation of antimicrobial therapy, since, in several clinical trials, patients with PCT values below the cut-off point for this serum marker and in whom antimicrobial treatment has not been initiated have favorable outcomes, while other patients with the same characteristics do not have a similar clinical course.
Other factors were considered that, based on the experience of the consensus group's experts, do not support the use of this test in this context, including cost, the fact that it is not readily available at the various levels of health care, and the typical delay in reporting the results in some health care centers (up to 72 hours).
Summary of evidence
Several studies have attempted to establish a reliable cut-off point for the serum PCT level to differentiate a CAP of viral etiology from one of bacterial origin, but so far it has not been possible to establish a standard threshold, although it is clear that the higher the value, the more likely it is that the origin is bacterial.41 For instance, the study conducted in Switzerland by Christ-Crain et al 42 in 243 patients (35.40% with CAP) found that the adjusted relative risk (RR) of antibiotic exposure was 0.49 (95%CI: 0.44-0.55; p<0.0001) in the group in which the PCT test was used (n=124) compared to the standard group (clinical trial only); however, other clinical outcomes such as mortality, length of hospital stay or development of complications were not evaluated.42 Likewise, a randomized controlled clinical trial conducted in Switzerland in 302 patients diagnosed with CAP reported that the use of PCT levels reduced antibiotic exposure (RR: 0.52, 95%CI: 0.48-0.55; p<0.01), antibiotic prescriptions on admission (85 vs. 99%; p<0.01), and duration of antibiotic treatment (median: 5 vs. 12 days; p <0.01), but, again, no prognostic clinical outcomes were assessed, and neither the length of hospital stay nor the risk of clinical failure.43
Regarding the evaluation of the impact of using this biomarker in terms of adverse effects, Schuetz et al, 44 in a randomized controlled clinical trial conducted in 1 359 patients with lower respiratory tract infection admitted to the emergency departments of 6 hospitals in Switzerland, 68.1% of whom had CAP, found that, considering the reduction in the duration of antibiotic treatment, patients in the PCT group (n=671) had a lower risk of adverse events associated with antibiotic use. However, concerning undesirable outcomes such as death, ICU admission, disease-specific complications, or recurrent infection requiring antibiotic use during the last 30 days, the use of a PCT algorithm was not superior to the use of treatment strategies based on clinical judgment.
Furthermore, the limitations for its implementation in Colombia should be taken into account, since the PCT test is not available in all health care centers and its use is widely limited because it is not included in the health benefits plan. Consequently, given the importance of initiating antimicrobial treatment in a timely manner, its use could entail a delay in the initiation of antibiotic administration in patients who require it.
Question N° 7. What is the best strategy for empirical antimicrobial therapy in adult outpatients with CAP?
Rationale for the recommendation
For this recommendation, the consensus group considered studies conducted in patients with CAP treated in the outpatient setting. To establish the first-line antimicrobial treatment, the most frequent etiological microorganisms in this population were considered; the proposed antimicrobial treatment schemes do not include coverage of atypical bacteria because no harmful outcomes have been reported in patients with CAP treated with amoxicillin. Another aspect in favor of the use of amoxicillin in monotherapy is that there are a large number of studies on the safety of this antimicrobial.
In cases in whom amoxicillin is contraindicated, such as patients with a history of allergy to this antibiotic, the consensus group recommended the administration of doxycycline or clarithromycin. These antimicrobials were not considered as first-line treatment for two reasons: first, the additional adverse effects of macrolides related, in particular, to electrocardiographic changes, arrhythmias, and increased risk of sudden death, and second, to promote the rational use of antimicrobials in this clinical setting.45
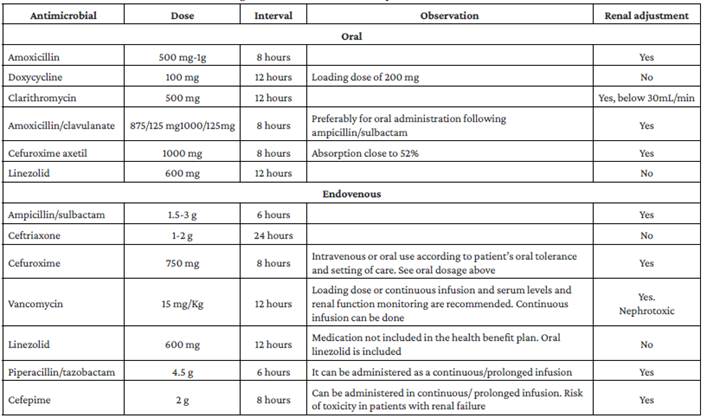
Finally, the use of this group of drugs for the treatment of CAP should be avoided as a good practice point bearing in mind that quinolone antibiotics increase the risk of inducing antimicrobial resistance in Gram-positive cocci and enterobacteria; the fact that they are used as second-line treatment for tuberculosis, an endemic disease in Colombia; and the frequency and severity of adverse effects related to the administration of these antibiotics.46,47 Prescription of antimicrobials should be based on the antibiotic susceptibility profile of the microorganisms causing CAP in each health care center. See Table 3 48-52 and Table 4.
Table 3 Frequency of microorganisms causing CAP in Colombia.
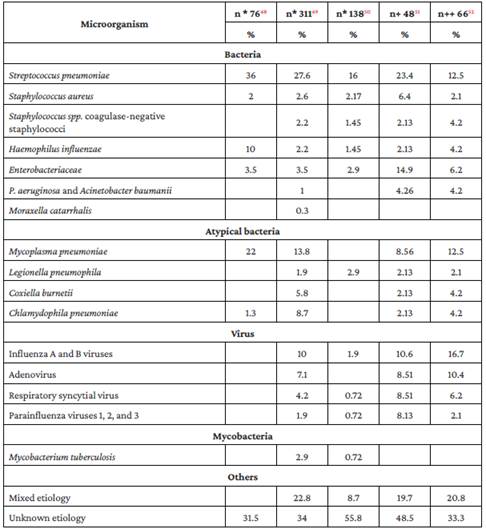
n*: number of patients.
n+: number of adults aged 65 and over.
n++ number of adults with severe CAP.
Source: Own elaboration.
Summary of evidence
Data on the superiority or non-inferiority of antimicrobials in patients with CAP receiving outpatient treatment are scarce, as documented by a Cochrane review conducted in 2014.53 For example, Maimon et al., 54 found no significant differences in terms of morbidity or mortality, despite the fact that some antimicrobials did not cover atypical bacteria, in a meta-analysis that included five studies comparing the usefulness of macrolides vs. fluoroquinolones in the outpatient treatment of CAP; three comparing macrolides vs. beta-lactams; three comparing fluoroquinolones vs. beta-lactams; and two comparing cephalosporins vs. beta-lactam beta-lactamase inhibitors.
Similarly, Bonvehi et al., 55 in a study conducted in Argentina in 327 outpatients with CAP comparing the use of clarithromycin (n=160) vs. amoxicillin+clavulanate (n=167), reported that there were no significant differences between groups in clinical and microbiological response rates, nor in the presence of adverse effects. On the other hand, studies that have evaluated the usefulness of amoxicillin in this clinical setting have reported that, compared to the use of other antimicrobials such as broad-spectrum quinolones, the use of high doses of this drug has not shown any significant difference in terms of clinical success and adverse effects.56,57
Information on the frequency of microorganisms causing pneumonia in Colombia is limited. In general, three groups of microorganisms have been described: classical bacterial agents with a high virulence potential, including S. pneumoniae, Haemophilus influenzae, Staphylococcus aureus; intracellular microorganisms, usually grouped as atypical, which include Mycoplasma pneumoniae, Chlamydophila pneumoniae, Legionella spp, and Coxiella burnetii; and viruses, most notably influenza viruses, respiratory syncytial virus, rhinoviruses, coronaviruses, adenoviruses and, recently, SARS CoV-2 in the adult population. Table 3 presents information on the relative frequency of the most frequent microorganisms reported by Colombian studies conducted in patients with CAP.
The frequency of Streptococcus pneumoniae isolates in Colombia, as well as the relative frequency of the different serotypes and their antimicrobial resistance, has varied after the introduction and extensive use of pneumococcal vaccines in the Expanded Program for Childhood Immunization. Although penicillin resistance decreased during the first few years after its inclusion, penicillin, ceftriaxone, and macrolide resistance rates in S. pneumoniae isolates have increased in recent years due to the selection of certain serotypes not covered by the vaccine.58
Furthermore, according to data from the National Public Health Surveillance System - SIVIGILA, since 2017 the proportion of isolates that are not sensitive to macrolides and penicillins or ceftriaxone in adults is greater than 25% and 10%, respectively.59 This suggests that epidemiological surveillance for this microorganism should be maintained in the country, although the impact of its resistance to antimicrobials is still unclear in the context of pneumonia,60 and more studies are needed in this regard.
Question N° 8. What is the best strategy for empirical antimicrobial treatment in adult inpatients with CAP?
Recommendations
Patients with moderate CAP:
We recommend using the following antimicrobial therapy in hospitalized adult patients with a diagnosis of moderate CAP and no risk factors for MRSA or P. aeruginosa (strength of recommendation: strong for; quality of evidence: moderate ⨁⨁⨁◯):
° Monotherapy with a beta-lactam (ampicillin/sulbactam) and re-evaluation 48 to 72 hours after treatment initiation to consider the need to add a macrolide (clarithromycin), taking into account aspects such as worsening of vital signs, ability to eat, and mental status.
In case the patient is allergic to penicillin, it is recommended to use (strength of recommendation: strong for; quality of evidence: moderate ⨁⨁⨁◯):
Patients with severe CAP:
We recommend using the following antimicrobial therapy in hospitalized adult patients with a diagnosis of severe CAP (Table 1) and no risk factors for MRSA or P. aeruginosa (strength of recommendation: strong for; quality of evidence: moderate ⨁⨁⨁◯):
If the patient is allergic to penicillin, we recommend using (strength of recommendation: strong for; quality of evidence: moderate ⨁⨁⨁◯):
Rationale for the recommendation
Recommendations for the treatment of CAP patients in the hospital setting are based on the treatment of the microorganisms that most frequently cause infection. There is no strong evidence on the benefits of using macrolides or antibiotics for the treatment of atypical bacteria in hospitalized patients with moderate CAP; studies comparing monotherapy with beta-lactam vs. beta-lactam with macrolide have not reported significant differences in that scenario. In the case of severe CAP, we recommend using a macrolide considering the coverage of atypical bacteria with high mortality burden such as Legionella and the additional effects of this group of drugs, such as modulation of inflammation. Furthermore, taking into account the quinolone resistance profile of the microorganisms that cause CAP and the adverse effects of these antibiotics, the use of this group of drugs for the treatment of these patients should be avoided as a good practice point. Antimicrobial prescription should be based on the antibiotic susceptibility profile of the microorganisms causing CAP in each health care center. See Tables 3 and 4.
Summary of evidence
Some studies have compared the use of beta-lactams with macrolides vs. monotherapy, although the latter with quinolones, and have reported that there are no differences between the two schemes; however, these studies have a low quality, small samples, and a non-inferiority design.61-63 Other research has evaluated the non-inferiority of beta-lactam and macrolide combination therapy vs. beta-lactam monotherapy, such as the open-label randomized clinical trial conducted by Garin et al. 64 in 580 patients with CAP hospitalized in 6 hospitals in Switzerland, which did not report a clear difference between arms (monotherapy arm, n=291 vs. combination therapy arm, n=289), but did suggest that patients with intracellular microorganisms took longer to achieve clinical stability.64
Nevertheless, the results reported by Garín et al. 64 differ from those described by Postma et al. 65 who compared various treatment strategies for CAP (beta-lactam monotherapy vs. fluroquinolone monotherapy vs. beta-lactam and macrolide combination therapy) in a cluster-randomized crossover clinical trial in 2 283 patients with mild to moderate CAP requiring hospitalization. The authors concluded that beta-lactam monotherapy was not inferior to the other antimicrobial treatment options for the outcomes of 90-day mortality and length of hospital stay, the latter being similar for all strategies.
These data, taken together, suggest that the benefit of using combination therapy in hospitalized patients with mild or moderate CAP is unclear, as its use does not offer any benefits in terms of reduced risk of progression to more severe forms of the disease and reduced length of hospital stay, but instead may contribute to increased antibiotics use, higher resource expenditure, and increased risk of adverse effects. As an alternative for the treatment of patients who are allergic to penicillin, the consensus group recommended the use of ceftriaxone, a therapy included in the non-inferiority studies of beta-lactam monotherapy referred to above. Likewise, as indicated in the systematic reviews and meta-analysis of Picard etal, 66 several clinical studies have evidenced the low frequency of cross-reactivity in allergic reactions in patients with a history of penicillin allergy who receive this drug. The next alternative in patients with a history of beta-lactam allergy is doxycycline, an antibiotic that has proven to be efficient due to its broad coverage of the causative microorganisms, as well as safe and cost-effective for the treatment of patients with mild to moderate CAP in the hospital setting.67
In patients with severe CAP, we recommend using beta-lactam plus macrolide to cover atypical bacteria such as Legionella, whose presence has been associated with a higher mortality rate, especially in older adults. In this regard, Nie et al, 68 in a meta-analysis that included 16 cohort studies (42 942 patients with CAP), reported a significant decrease in mortality in patients who received combination therapy (beta-lactam+macrolide) compared to those who received monotherapy (beta-lactam) (adjusted OR: 0.67, 95%CI: 0.61-0.73; p<0.01).
A similar finding was also described in the systematic review and meta-analysis (14 studies; 33 618 patients) by Horita et al.,69 specifically in patients with severe CAP, where the random-model meta-analysis yielded an OR for all-cause mortality for beta-lactam plus macrolide compared with beta-lactam monotherapy of 0.80 (95%CI: 0.69-0.92; p<0.01). Collectively, this evidence suggests the benefit of using combination therapy in patients with severe CAP, which is related either to antibiotic coverage of Legionella species that may be associated with increased mortality, or to the effect of combination therapy in patients with severe CAP caused by S. pneumoniae. 68
Question N° 9. What is the best strategy for empirical antimicrobial treatment in adult inpatients with CAP and with risk factors for MRSA or P. aeruginosa?
Recommendations
We recommend taking the following actions in hospitalized adult patients with a diagnosis of moderate or severe CAP and previous respiratory isolate of MRSA (strength of recommendation: strong for; quality of evidence: low ⨁⨁◯◯):
° Initiate antimicrobial therapy based on the microbiological report of the previous respiratory isolate AND
° Perform culture or PCR of upper respiratory tract specimen to adjust treatment or confirm the need for continued therapy based on results OR
° Perform culture or PCR of upper respiratory tract specimen to adjust treatment or confirm the need for continued therapy based on results AND initiate antimicrobial therapy with vancomycin or linezolid.
We recommend taking the following actions in hospitalized adult patients with a diagnosis of moderate or severe CAP and previous respiratory isolate of P. aeruginosa (strength of recommendation: strong for; quality of evidence: low ⨁⨁◯◯):
° Initiate antimicrobial therapy based on microbiological report of previous respiratory isolate AND
° Perform culture or PCR of upper respiratory tract specimen to adjust treatment or confirm need for continued therapy based on results OR
° Perform culture or PCR of upper respiratory tract specimen to adjust treatment or confirm the need for continued therapy based on the results AND initiate antimicrobial therapy with piperacillin/tazobactam or cefepime.
We suggest taking the following actions in hospitalized adult patients with a diagnosis of moderate CAP and a history of hospitalization and/or antimicrobial therapy in the past 90 days or with locally validated risk factors for MRSA or P. aeruginosa (strength of recommendation: weak for; quality of evidence: low ⨁⨁◯◯):
° Perform culture or PCR of upper respiratory tract specimen AND
° Initiate antibiotic treatment covering MRSA or P. aeruginosa only if results are positive.
We recommend taking the following actions in hospitalized adult patients with a diagnosis of severe CAP and a history of hospitalization and/or antimicrobial therapy in the past 90 days or with locally validated risk factors for MRSA (strength of recommendation: strong for; quality of evidence: low ⨁⨁◯◯):
° Perform culture or PCR of upper respiratory tract specimen to adjust treatment or confirm the need for continued therapy based on results AND initiate antimicrobial therapy with vancomycin or linezolid, along with measurement of vancomycin levels, if appropriate.
We recommend taking the following actions in hospitalized adult patients with a diagnosis of severe CAP and a history of hospitalization and/or antimicrobial therapy in the past 90 days or with locally validated risk factors for P. aeruginosa (strength of recommendation: strong for; quality of evidence: low ⨁⨁◯◯):
Rationale for the recommendation
There are no clinical studies on predictors of MRSA or P. aeruginosa infection and there are no predictive scores to determine their presence. This, added to the fact that the prevalence of these microorganisms varies widely in each region and health care center in the country, led the consensus group to recommend the use of antimicrobials that cover MRSA or P. aeruginosa in patients with moderate to severe CAP and with a history of isolate of these microorganisms, as well as to perform culture or PCR of upper respiratory tract samples in order to establish a targeted treatment that promotes the rational use of antimicrobials.
The other clinical setting where we recommend antibiotic treatment of these bacteria is in patients with severe CAP and a history of hospitalization and/or antimicrobial therapy in the past 90 days or with locally validated risk factors for the presence of MRSA or P. aeruginosa. The strength of this recommendation is weak, and the evidence has a low quality because no studies were found to support the increased risk of MRSA or P. aeruginosa infection in the presence of these factors; however, due to the high mortality of patients with severe CAP, the use of antibiotics covering these bacteria should be considered in this clinical setting.
Summary of evidence
The main risk factor for suspecting infection by P. aeruginosa or MRSA is a history of infection or colonization by one of these microorganisms; in this regard, for example, an OR of 6.21 (95%CI: 3.25-11.85) has been described for MRSA.70 Other risk factors include the presence of respiratory comorbidities (OR: 5.8, 95%CI: 2.2-15.3; p<0.01), having a history of hospitalization (OR: 3.8, 95%CI: 1.8-8.3; p=0.02),71 especially in the last 90 days, and the use of antimicrobials in the last 90 days (OR: 2.90, 95%CI: 1.13-7.45; p=0.02).72 An additional risk factor that should be considered to initiate antibiotic coverage is the epidemiology of these infections in the health care center, which should include the local antimicrobial susceptibility profile.
In patients with moderate or severe CAP and in whom, depending on the presence of risk factors, MRSA or P. aeruginosa infection is suspected, cultures or PCR of respiratory samples should be performed to confirm this etiology and adjust antimicrobial treatment. This is explained by the fact that antimicrobial stewardship is relevant, as it has been shown to be safe and to reduce the duration of antibiotic treatment, the length of hospital stay, and the frequency of complications related to adverse effects of broad-spectrum antimicrobials.73
In this regard, Cremers et al. 74 found that, compared to no adjustment, adjusting antimicrobial therapy reduced mortality in patients with bacteremic nemococcal CAP (adjusted OR: 0.35, 95%CI: 0.12-0.99). Furthermore, Carugati et al., 75 in a study conducted with data from 261 hospitalized patients with CAP bacteremia, reported that adjustment of antimicrobial therapy (de-escalation) based on microbiological test results did not increase the risk of clinical failure in comparison with patients in whom antimicrobial therapy was de-escalated (RR: 0.89, 95%CI: 0.63-1.27; p=0.54). Nevertheless, in a recent large cohort study (88 605 hospitalizations due to CAP in the Veterans Affairs health care system medical centers), inverse propensity score-weighted regression of the weights showed that empirical use of linezolid or vancomycin in these patients did not decrease mortality but, on the contrary, increased it. 76
The costs of generic antimicrobials in Colombia are quite low and it is possible that the recommended therapeutic schemes are not expensive, both for intravenous and oral therapy. However, as mentioned above, formal evaluations of the cost-effectiveness of recommended treatments are needed in the region.
Question N° 10. What is the appropriate duration of antimicrobial therapy in adult patients with CAP, both outpatients and inpatients?
Recommendations
We recommend that the duration of antimicrobial therapy should not exceed 5 days in total and considering the following criteria for discontinuation: clinical stability of the patient (resolution of alterations in vital signs [heart rate, respiratory rate, blood pressure, oxygen saturation, and body temperature]), adequate tolerance to oral administration of food and fluids, and no alteration in mental status (strength of recommendation: strong for; quality of evidence: moderate ⨁⨁⨁◯).
We recommend establishing the duration of antimicrobial therapy depending on the patient's clinical course, microbiological test results, and biomarker levels (strength of recommendation: strong for; quality of evidence: moderate ⨁⨁⨁◯).
Good clinical practice point
If available, serum PCT testing may be used to determine whether to discontinue antimicrobial therapy in hospitalized patients.
Rationale for the recommendation
Taking into account that, in general, adequate antimicrobial prescription leads to clinical stability of patients 48-72 hours after starting treatment, we recommend a maximum duration of antimicrobial therapy of 5 days in patients with mild to moderate CAP and that discontinuation be decided based on clinical judgment, which includes criteria such as the absence of alterations in vital signs, adequate tolerance to oral administration of food and fluids, state of consciousness, and absence of complications. In cases of severe CAP, the duration of antimicrobial therapy should be established based on medical judgment, taking into account clinical and microbiological factors and monitoring of biomarkers such as serum PCT, if possible.
Summary of evidence
Historically, studies on the duration of antimicrobial treatment in patients with CAP have been grouped into two classes: a duration <7 days and a duration >7 days. The studies identified, such as that performed by el Moussaoui et al. 77 in 119 patients with CAP, compare the use of amoxicillin, initially intravenously for 3 days and then orally for 5 more days, with the use of placebo in patients with mild to moderate CAP, concluding that although both strategies showed similar results in the outcomes of resolution of symptoms and changes in x-ray findings, the frequency of adverse effects was greater in the group of patients in which antibiotics were administered for 7 or more days (21% vs. 11%).
These results have been confirmed by other studies such as the one conducted by Dunbar et al. 78 in 528 patients with mild to moderate CAP in which there was no statistically significant difference in clinical response rates or microbiological eradication rates between the use of levofloxacin for 5 days vs. the use of this drug for 10 days. Likewise, besides not finding discordant results between the duration of antibiotic treatment (7 days vs. 10 days) in hospitalized patients with severe CAP in terms of late recurrences or cure rates, Siegel et al. 79 reported a shorter length of hospital stay, a lower frequency of adverse effects, and lower costs in the group of patients who received antibiotic treatment with a shorter duration.
It should be noted that the use of scores to evaluate the possibility of implementing antimicrobial treatments of even 3 days has also been suggested, since these scores, some of which have been translated into and culturally adapted to Spanish,80 take into account the symptoms and the subjective clinical evolution of the patient to define the time of treatment.
Discontinuation of antimicrobial therapy should be based on the clinical stability of the patient, understood as the presence of normal vital signs, adequate tolerance to oral administration of food and fluids, and the absence of altered mental status; in this sense, these findings are expected to be observed in patients with mild to moderate CAP 2 to 3 days after initiation of antimicrobial therapy.81,82 Furthermore, the assessment of patients' clinical condition has been shown to reduce the length of hospitalization and duration of antimicrobial therapy, with no adverse impact on patient safety.83
Finally, it should be pointed out that there is currently no evaluation of the cost-effectiveness of the proposed interventions, although it is expected that they will be cost-effective, considering that they have the potential to reduce the length of hospital stay and, therefore, the possible associated complications, which would imply a reduction in the costs associated with the care of these patients since their use would allow early hospital discharge.
Question N° 11. What is the appropriate route of administration of antimicrobial therapy in adult patients with moderate to severe CAP?
Recommendations
We recommend using oral antimicrobials as first-line treatment in adult patients with moderate to severe CAP, always taking into account tolerance to oral administration of food/drugs/fluids and severity of infection (strength of recommendation: strong for; quality of evidence: very low ⨁◯◯◯).
We recommend reassessing the patient's clinical condition 48 hours after starting intravenous antimicrobial therapy to consider switching to oral antimicrobials (strength of recommendation: strong for; quality of evidence: very low ⨁◯◯◯).
Rationale for the recommendation
Initially, the route of administration of antimicrobials in patients with moderate to severe CAP was exclusively intravenous for the entire course of treatment. However, more recent studies have promoted the use of oral antimicrobials considering the decrease of adverse effects, the convenience of this route of administration, and the effectiveness of oral antimicrobial treatment, particularly in cases where 48 hours of intravenous therapy have already been completed and the patient is clinically stable. Therefore, the consensus group accepted and included this recommendation, as it encourages the rational use of antimicrobials, reduces the costs of care and hospital stay and, according to the evidence found, is a safe decision.
Summary of evidence
The evidence identified regarding the route of administration of antimicrobials in patients with moderate to severe CAP has a very low quality. For example, in a literature review, Cassiere & Fein84 report that switching from intravenous to oral administration in low-risk patients was an effective and safe intervention in the treatment of CAP. Likewise, Marras et al.,85 in a meta-analysis that included 7 studies (a total of 1 366 hospitalized adult patients with CAP), concluded that in patients with non-severe CAP who received oral antibiotic treatment, the strategy was effective and therapy lasted 1.3 days less (95%CI: 0.4%-2.2% days; p<0.01) than in the group of patients who received intravenous therapy.
Furthermore, based on the evidence found, the main costs of treating moderate to severe CAP are related to the duration of intravenous antimicrobial therapy and the length of hospital stay, so switching to oral antibiotics significantly reduces the costs of care for these patients, without this change implying harm to the patient or being associated with recurrences of infection and the development of complications.86 Once the patient is clinically stable and the switch from intravenous to oral antibiotics has been made, in-hospital observation is not required, so discharge, if there are no other contraindications, can be immediate, as confirmed by Nathan et al. 87 in their retrospective study carried out with data obtained from the US Medicare National Pneumonia Project. In Colombia, the antimicrobials mentioned in the recommendations are covered by the health benefits plan of the Colombian health system.
Question N° 12. Should antiviral therapy be included in the treatment of adult patients with CAP who test positive for influenza?
Rationale for the recommendation
Evidence on the use of influenza antiviral therapy in the context of CAP is very scarce and has a very low quality. No clinical trial that directly evaluated the usefulness of this type of treatment exclusively in patients diagnosed with CAP was found, but instead evidence comes from observational studies conducted in patients with different types of lower respiratory tract infections describing benefits in reducing the frequency of complications, the duration of symptoms, and the risk of mortality, even when antiviral administration is initiated more than 48 hours after the onset of symptoms. However, these results have not been consistent and have only been reported for patients with severe CAP. Moreover, the benefits of using these drugs have only been described in patients with a positive microbiological test for influenza virus, so the consensus group decided to make this recommendation only in this setting.
Summary of evidence
No clinical trials evaluating the use of oseltamivir in patients with CAP were found; in fact, very few studies were identified, and they have a very low quality with a high risk of bias. For example, Lee et al. 88 reported that for hospitalized patients with confirmed influenza A/B respiratory infection (n=754), the use of antiviral drugs was significantly associated with a reduced risk of mortality (adjusted HR: 0.27, 95%CI: 0.13-0.55; p<0.01) and that early treatment (within 2 days after symptom onset) was associated with faster discontinuation of oxygen therapy (adjusted HR: 1.30, 95%CI: 1.01-1.69; p=0.04) and sooner hospital discharge (adjusted HR: 1.28, 95%CI: 1.04-1.57; p=0.01). McGeer et al.,89 in a study conducted in Toronto involving 512 hospitalized patients with a laboratory-confirmed diagnosis of influenza (185 children, none of whom died), reported that treatment with influenza antiviral drugs was associated with a significant decrease in mortality (OR: 0.21, 95%CI: 0.06-0.80) but that no benefit was observed for other outcomes such as length of hospital stay.
According to some of the initial clinical studies on this subject, such as the one conducted in the United States by Siston et al. 90 with data from 788 pregnant women diagnosed with AH1N1 influenza, antiviral therapy should be initiated within 48 hours of symptom onset.90 However, good results have also been reported when this therapy is started 4 to 5 days after symptom onset.91
Although the usefulness of antiviral therapy has been reported primarily in the in-hospital setting, a reduction in the risk of complications has also been demonstrated in the outpatient setting. This was evidenced in a systematic review and meta-analysis that included 9 clinical trials (4 328 patients) where it was reported that the frequency of lower respiratory tract complications requiring antibiotics at 48 hours was lower in the group that received oseltamivir compared to the placebo group (4.2% vs. 8.7%), estimating a 44% reduction in the risk of these complications with treatment with this antiviral (RR: 0.56, 95%CI:0.42-0.75; p=00001), with an absolute risk difference of -3.8% (95%CI: -5.0 to -2.2).92 Concerning the in-hospital setting, Muthuri et al.,93 in a systematic review including individual data from 29 234 patients infected with H1N1pdm09 influenza virus and hospitalized during the 2009 pandemic, found that, compared with using no antiviral therapy, treatment with neuraminidase inhibitors (regardless of the time of onset) was associated with a reduction in the risk of mortality (adjusted OR: 0.81, 95%CI: 0.70-0.93; p=0.0024). Also, compared to late onset, early initiation of treatment (within the first 2 days after symptom onset) was also significantly associated with a reduction in the risk of mortality (adjusted OR: 0.50, 95%CI: 0.37-0.6; p<0.0001).
The use of oseltamivir and zanamivir (another neuroaminidase inhibitor) for the treatment of influenza has been extensively studied in the outpatient setting; in this regard, it has been reported that although these antiviral drugs mildly reduce symptoms, their use does not have a relevant clinical impact and their effect on reducing the use of antibiotics is low or null.94 Furthermore, a systematic review that included randomized controlled clinical trials conducted in patients with confirmed or suspected exposure to influenza virus reported that, although the use of oseltamivir reduced the time to first symptom relief in adults by 16.8 hours (95%CI: 8.4-25.1 hours; p<0.001), this drug was associated with an increase in the frequency of nausea, vomiting and psychiatric symptoms,95 thus concluding that its use in the outpatient setting is not very beneficial for these patients. In view of the above, the need to use this drug only in patients with a microbiologically confirmed diagnosis of influenza is strongly emphasized.
In Colombia, during the 2009 H1N1 influenza pandemic, the use of oseltamivir was implemented for the treatment of patients with severe acute respiratory infection, so the access to this drug is guaranteed since then in all health centers of the country. This fact makes this recommendation feasible with a limited impact on the costs currently incurred by healthcare centers associated with the care of these patients.96
Question N° 13. Should corticosteroids be used in adult inpatients with CAP?
Recommendations
We do not recommend using corticosteroids to treat patients with mild to moderate CAP (strength of recommendation: strong against; quality of evidence: moderate ⨁⨁⨁◯).
Patients with severe CAP (Table 1):
We suggest using corticosteroids in patients with severe CAP (strength of recommendation: weak for; quality of evidence: moderate ⨁⨁⨁◯).
Rationale for the recommendation
No studies were found to support the use of corticosteroids in patients with mild to moderate CAP; however, there is scientific evidence of the benefit of their use in patients with severe CAP in terms of mortality, requirement of mechanical ventilation, and length of stay in the ICU.
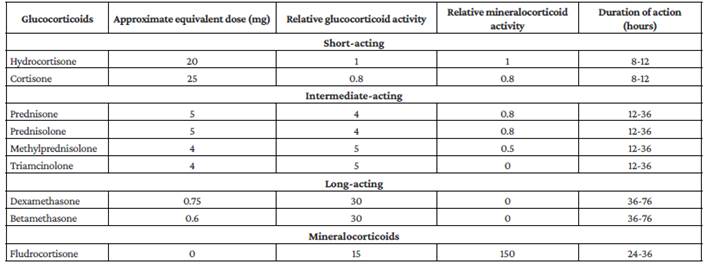
Therefore, we suggest using these drugs in these patients taking into account clinical judgment, the absence of contraindications, and the analysis of the balance between risks and benefits, since their use is frequently associated with adverse effects, hyperglycemia being the most common, which could affect the clinical outcome of patients with sepsis of pulmonary origin. Corticosteroids and suggested doses to be used in these patients are presented in Table 5.97,98
Summary of evidence
Some studies have documented the benefit of using corticosteroids in patients with severe CAP. Confalioniere et al., 99 in a multicenter randomized controlled trial conducted in 46 patients with CAP admitted to the ICU and receiving antibiotic treatment, found that, compared to placebo, the use of hydrocortisone infusion was associated with a significant decrease in hospital stay and mortality. However, it has not been possible to reproduce this considerable reduction in the risk of death of these patients in other studies and even some studies, such as the multicenter, randomized, double-blind, controlled trial conducted by Torres et al. 100 in 120 patients with severe CAP, have described that the use of corticosteroids did not lead to a decrease in hospital stay or mortality.
At first, different meta- analyses, such as the one conducted in 2015 by Chen et al. 101 (7 randomized clinical trials; 944 patients with CAP), documented that the use of these drugs (specifically glucocorticoids) was associated with a decrease in hospital stay, but more recent meta- analyses, such as the one conducted by Jian et al 102 (10 randomized clinical trials; 665 patients), have identified a significant reduction in mortality. In addition, other studies, such as the multicenter double-blind randomized controlled trial conducted by Blum et al.103 in 785 hospitalized adult CAP patients, have documented that the time to reach clinical stability is shorter in patients receiving steroids such as prednisone and that the use of these drugs is also associated with a reduction in the time requiring mechanical ventilation.
The use of steroids has been associated with important adverse effects such as alterations in glucose metabolism (hyperglycemia) and gastrointestinal bleeding. However, the clinical studies reviewed, at the doses and times recommended in this CPG, did not report an increase in the frequency of these events.102 Nevertheless, in the case of patients with pulmonary sepsis, the presence of adverse effects should be routinely evaluated, and appropriate measures should be taken for their prevention and timely control.
On the other hand, the use of steroids is a standard practice for the treatment of patients with COVID-19, so, provided there is no contraindication, these drugs should be used in these patients.9
Finally, according to a recently published meta-analysis that included 21 observational studies, corticosteroid use was associated with increased mortality in patients with influenza (OR: 3.9, 95%CI: 2.3-6.6, 15 studies; adjusted HR: 1.49, 95%CI: 1.09-2.02, 6 studies).104
Implementation and update of the CPG
This CPG should be implemented in health centers that provide health care services to adult patients with CAP in order to support activities related to the treatment of this population and programs to optimize the use of antimicrobial agents. Similarly, the indicators presented in Table 6 should be considered to measure the progress of the guideline's implementation, with the frequency and reporting requirements that each institution deems pertinent.
Table 6 Indicators for measuring the implementation of the clinical practice guideline.
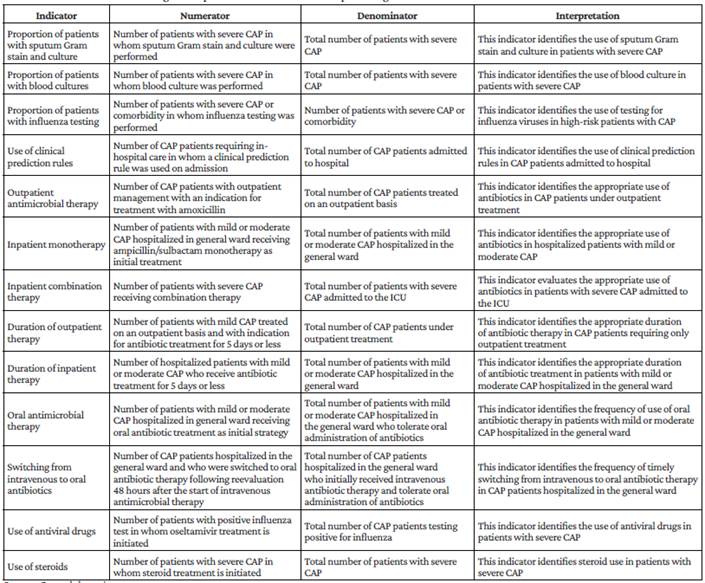
Source: Own elaboration.
In order to facilitate the implementation of this CPG, the following dissemination tools will be used to provide the country's health professionals with better access to its contents: publication of the guideline in the Revista de la Facultad de Medicina of the Universidad Nacional de Colombia and on the website of the Asociación Colombiana de Infectología, as well as inclusion of the guideline recommendations in the contents of a massive open online course (MOOC) and a mobile application for CAP management.
Finally, it is considered that this CPG should be updated within a period no longer than 5 years, following the same methodology and rigor that was used for its development. The topics to be addressed may be reconsidered depending on the needs arising from clinical practice or the appearance of new evidence that should be included in the CPG.











 texto em
texto em