Introduction
After a total gastrectomy, intestinal transit must be restored, with Roux-en-Y esophago-jejunostomy being the most commonly used technique for this. However, it should be borne in mind that multiple complications, such as esophagojejunal anastomosis fistulas, can occur following this type of intervention and they can be life-threatening.1
In general, an anastomotic leak is a serious complication that can lead to death. Its frequency varies depending on various factors such as the characteristics of the institution where the patient is treated (surgical volume), the experience of the surgeon performing the procedure, nutritional status, underlying comorbidities of the patient, among others. In the case of esophagojejunal anastomosis fistulas, it has been reported that mortality rates are variable (5% to 60%) and that their occurrence implies prolonged hospital stays and, therefore, a significant increase in the costs of care for these patients.2-5
Fistulas or intestinal leaks, regardless of their etiology, are in most cases a difficult medical problem6 that usually requires various therapeutic interventions. The most important aspect for proper treatment of these complications is to consider the patient's clinical condition, so it is recommended to individualize the treatment based on their needs, while always attempting to preserve intestinal continuity.7
Depending on the extent of the fistula and the patient's clinical condition, several therapeutic alternatives are available, including conservative treatments -such as parenteral or enteral nutrition by means of tube distal to leak, abdominal drainage, and administration of antibiotics- or minimally invasive or invasive procedures such as endoscopy and reoperation.8,9
Over the past decade, non-surgical approaches have been the most widely used in the treatment of upper gastrointestinal defects because, for example, endoscopic treatment offers both a diagnostic and a therapeutic approach.10 This feature, along with the benefits of vacuum-assisted closure (VAC) therapy, has allowed the development of a new approach to treating such defects.
The following is the case of a patient who received endoscopic VAC therapy to treat a persistent esophagojejunal anastomosis fistula.
Case presentation
72-year-old man who was diagnosed with gastric adenocarcinoma of the body and fundus in August 2018. In January 2019, he underwent a total gastrectomy with D2 lymphadenectomy and a Roux-en-Y anastomosis with curative intent in a quaternary care hospital in Popayan, Colombia. The patient had a history of hypertension, type 2 diabetes mellitus, and morbid obesity.
On the sixth postoperative day, the patient presented with systemic inflammatory response syndrome and acute abdomen due to an esophagojejunal anastomosis fistula. As a result, he was taken back to surgery, finding peritonitis and intestinal material in the peritoneal cavity, for which reason cavity lavage was performed and an abdominal drainage tube was inserted. The cavity lavage and drainage of intestinal contents were repeated 48 hours later; however, 96 hours after the initial lavage (when the infection was apparently under control), he developed frozen abdomen, making it impossible to access the esophagojejunal anastomosis. Therefore, an assessment from the endoscopic surgery group was requested.
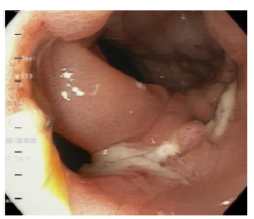
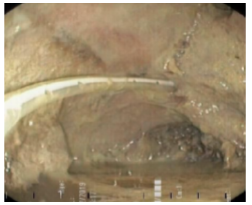
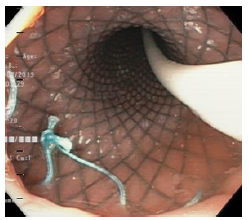
Twelve days after the gastrectomy, a first upper gastrointestinal tract endoscopy (EGD) was performed, which confirmed the clinical suspicion of leakage through the esophagojejunal anastomosis, with a dehiscence of 20% of the circumference (Figure 1). Consequently, treatment was initiated by inserting a self-expandable metallic stent (SEMS) (Figure 2).
Source: Image obtained during the study.
Figure 1 Dehiscence of esophagojejunal suture (anastomotic leak).
Source: Image obtained during the study.
Figure 2 Self-expandable metallic stent and nasojejunal tube.
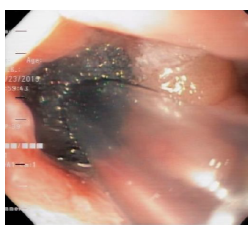
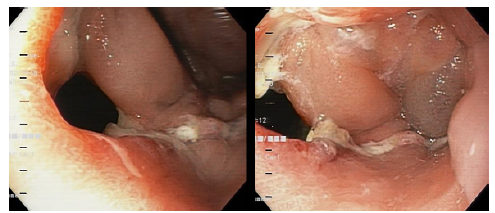
The patient's course was not satisfactory, as the leakage of intestinal contents through the abdominal drainage tube persisted. Eight days after inserting the SEMS, a second EGD was performed, in which leakage of intestinal contents between the distal end of the SEMS and the lifted jejunum was observed, as well as persistence of the esophagojejunal leakage. Therefore, the SEMS was removed, and an examination of the abdominal cavity was performed through the defect, finding localized abscess, cellular detritus, fibrin particles attached to cavity walls, and abdominal drainage tube (Figures 3 and 4).
Given the patient's condition, salvage therapy was started by endoscopic drainage of the intra-abdominal abscess found, the VAC system was placed endoscopically (Figure 5), and a nasojejunal tube was subsequently placed and enteral nutrition was initiated.
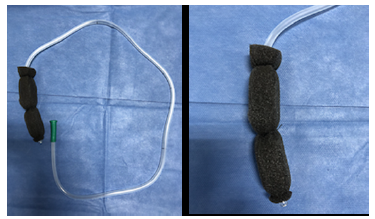
Since it is not yet possible to obtain an endoscopic kit for VAC therapy in Colombia, it was necessary to make a handmade kit (Figure 6), using a Levin No. 18 nasogastric tube with several additional fenestrations at its tip and a 1.5x1.5x5cm rectangular prism of black open-cell polyurethane foam (extracted from an abdominal VAC system foam) with a central longitudinal hole through which the probe tip was passed. The foam was fixed to the probe in three parts with 0-gauge silk suture and its corners were rounded with scissors.
Source: Image obtained during the study.
Figure 6 Handcrafted vacuum-assisted closure device made from polyurethane foam.
To position the foam of the VAC system inside the peritoneal cavity, a placement wire from an endoscopic gastrostomy kit was used, which was inserted into the abdominal drainage tube, recovered via endoscopy, and taken to the patient's mouth, prior to removal of the drainage tube. This wire was attached to the tip of the probe and the VAC system was mobilized by traction into the peritoneal cavity, where it was placed in the appropriate position.
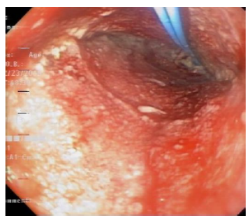
Subsequent VAC system replacements, which were carried out every 72 hours (for a total of 5 replacements), were performed in the same way, changing only foam length and probe diameter; the latter was connected to a negative pressure system intermittently at -125 mmHg. With each replacement, greater cleaning and reduction in the size of the peritoneal cavity were observed (Figure 7).
Source: Image obtained during the study.
Figure 7 First replacement of the handcrafted vacuum-assisted closure system.
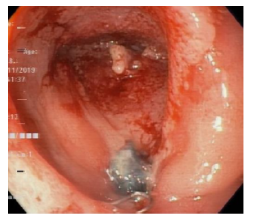
After 18 days of initiation of VAC therapy, complete drainage of the intra-abdominal abscess, closure of the peritoneal cavity, and more than 90% closure of the esophagojejunal fistula were achieved (Figure 8).
Source: Image obtained during the study.
Figure 8 Small residual diverticular formation measuring 22x18x25mm.
The patient's condition improved progressively, with resumption of oral intake 20 days after initiation of VAC therapy. Moreover, no new abdominal complications were observed during the follow-up period (17 months). The histopathological study of gastric lesion confirmed well-differentiated adenocarcinoma, classified as T2N0M0 and with involvement up to the muscularis propria. There was no need for adjuvant therapy.
Discussion
Endoscopic therapy of anastomotic leaks or intestinal perforations has become a safe and effective procedure in recent years, with the insertion of SEMS being the most commonly used method due to its efficacy of more than 60%.5,11-13 However, there are currently several treatment options available, and endoscopic vacuum-assisted wound closure is regarded as an innovative and effective method.13
In this regard, Rausa et al. 14 conducted a systematic review and meta-analysis in which they included studies comparing the use of SEMS with VAC therapy for the treatment of esophageal perforations and found that the rate of closure of these defects is significantly higher with VAC therapy than with SEMS (95%CI: 2.11-14.88; p<0.001), and that VAC therapy is associated with a shorter duration (95%CI: 6.6-1.4; p=0.021) and a lower rate of major complications (95%CI: 0.11-1.27; p=0.011) and hospital mortality (95%CI: 0.13-0.81; p=0.002) than SEMS placement. As a result, the authors concluded that, while SEMS is a viable alternative to surgery in the treatment of anastomotic leakage or intestinal perforation, with an overall success rate ranging from 44% to 88% of patients, endoscopic VAC therapy emerges as an additional therapeutic option, showing overall success rates that vary between 84% and 100%, as demonstrated in the case reported here, in which the patient did not have a satisfactory course with the placement of the SEMS, but did with endoscopic VAC therapy.
Negative pressure therapy was first used to treat wounds in the extremities complicated by infection or loss of soft tissues in the mid-twentieth century,15 but in recent decades, its use has spread to other areas, and it is now used by many medical specialties for the management of complex wounds, both external and internal, where endoscopic access is possible.
The mechanism of action of VAC therapy allows to improve the microenvironment of the wound or lesion being treated, facilitating healing through multiple molecular, vascular, and cellular processes. These processes include wound contraction (macro-deformation), stabilization of the wound environment, reduction of edema, removal of exudates and cellular detritus, micro-deformation of the surface, and an increase in local blood flow of up to four times, which also allows for increased angiogenesis, granulation tissue formation, and local bacterial count reduction.16-17
The principle by which VAC works had already been successfully implemented in the treatment of endoscopic access to anastomotic leaks from the upper and lower digestive tract. For example, Loske et al. 18 in a case series conducted in 14 patients with the full range of possible esophageal defects, found that complete healing of the esophageal defect was achieved in 13, with the other patient dying due to fulminant pseudomembranous colitis that developed while the esophageal defect was nearly healed.
For their part, Bludau et al. 19 published a study of 77 patients with upper digestive tract defects treated with VAC therapy between October 2010 and January 2017 (6 patients with spontaneous esophageal perforations, 12 with iatrogenic lesions, and 59 with post-surgical fistulas) and found that 77.9% of participants achieved a complete resolution of the defect. Said study demonstrates the great benefits of this novel system, such as fistula closure and infection control.
The duration of VAC therapy for successful closure of intestinal fistulas varies depending on the size of the defect and cavity contamination; however, Banasiewicz et al., 20 in a case series involving 3 critically ill patients with complex postoperative wounds complicated by multiple fistulas reported successful results within 29 to 35 days, during which 1 to 21 replacements were performed. The patient in the case reported here required 5 system replacements (performed over 18 days), which resulted in 90% closure of the lesion, removal of the enteral nutrition tube (which was maintained in situ throughout the treatment), and resumption of oral intake after removing the last foam, with excellent tolerance and evolution.
Conclusions
Endoscopic VAC therapy is an innovative method that has become a safe and effective alternative for the management of anastomotic fistulas and similar lesions of the upper and lower digestive tract. Furthermore, according to published studies, this is a reproducible procedure that is well tolerated by patients and significantly reduces morbidity and mortality.14