Introduction
With the emergence of MERS-CoV in 2012 and SARS-CoV in December 2019, global attention has focused on the coronavirus family, since these are major pathogens that cause respiratory tract infections of variable severity.1 The current public health crisis began in late 2019 with an unexplained increase in cases of pneumonia of unknown origin in Wuhan, China, which quickly spread to other cities and countries. Subsequently, in January 2020, SARS-CoV-2 was identified as the causative microorganism of this new disease, which was named Coronavirus Disease 2019 (COVID-19), and declared as a public health emergency of international concern on January 30 and as a pandemic on March 11, 2020, by the World Health Organization (WHO) due to its high pathogenic potential and rapid spread.2 Since its detection in China in December 2019 until March 9, 2021, 116 736 437 confirmed cases of COVID-19 were reported globally, of whom 2 593 285 died.3
At present, there is increasing research reporting that coronavirus infections do not only affect the respiratory tract. In this regard, it has been pointed out that central nervous system involvement may take place in susceptible individuals and contribute to the increased morbidity and mortality of severe COVID-19. Accordingly, it has been established that this disease not only leads to respiratory involvement, but can also affect the nervous system.4,5
As reported by Losy et al.,4 the first retrospective study on neurological symptoms in COVID-19, conducted in China by Mao et at., found that 36.4% of the 214 COVID-19 patients evaluated had neurological manifestations such as dizziness, headache, altered consciousness, impaired smell and taste, stroke, seizures, ataxia, and musculoskeletal injury, among others. Furthermore, other studies have reported the occurrence of anosmia, seizures, acute ischemic stroke, viral meningoencephalitis, acute necrotizing encephalopathy, acute flaccid paralysis, post/para-infectious syndromes, and corticospinal weakness in patients with severe COVID-19.6,7
Although the pathophysiological mechanisms underlying the involvement of the nervous system during SARS-CoV-2 infection have not been fully elucidated, evidence suggests that this disease could be a multifactorial phenomenon in which processes such as direct involvement, autoimmune factors, inflammation (cytokine storm), anterograde or retrograde axonal transport, pharmacological side effects, metabolic alterations, and neuropathy in the critically ill patient, among others, stand out.4,8-10
Taking into account the above and based on the need to offer patients with COVID-19 a more comprehensive approach to establish a diagnosis and timely treatment, the objective of this literature review was to collect and synthesize scientific evidence published within six months after the declaration of the COVID-19 pandemic on neurological manifestations in patients infected with SARS-CoV-2, as well as their variations and frequency in specific populations.
Materials and methods
A literature review was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.11 The search was performed in PubMed/ Medline using the following search strategy: study types: any type describing neurologic manifestations in patients with COVID-19; publication period: March 11 to August 31, 2020; publication language: English; search terms and equation: (("COVID-19") AND "Neurologic Manifestations") (MeSH terms).
The search, identification and selection process is described in detail below (Results section). All authors contributed to the review of the articles identified in the literature search, led by DLMA and EPCM, specialists in neurology and child neurology, respectively. It should be noted that during the title and abstract review stage, studies that did not address the topic of interest of the review were discarded. Moreover, during the full-text reading stage, studies were excluded if it was not possible to access the full text using institutional resources, as well as the following study types: narrative and systematic reviews, meta-analyses, and reflection articles. It is also necessary to point out that the review protocol was not registered.
The following data were extracted from the analysis of the articles included in the review: main neurological manifestation, secondary complications reported, sociodemographic variables of the patients, presence of comorbidities, and COVID-19 severity (presence of symptoms, requirement for hospitalization, etc.)
Data collected from individual case reports and case series were grouped by reported neurological manifestation and variables of interest to this study (age, sex, presence of comorbidities, and COVID-19 severity), and summarized in a table (available in the Results section). In said table, qualitative variables are presented as relative frequencies and medians with their respective interquartile ranges for qualitative and quantitative variables, respectively.
Results
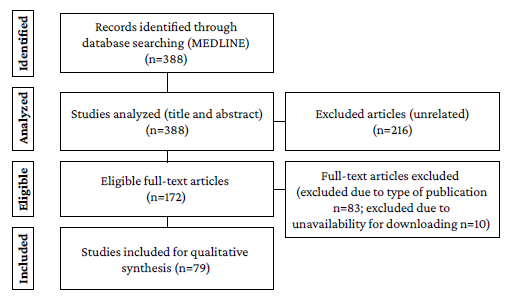
The initial search yielded 388 records, of which 216 were excluded during the title and abstract review stage because they did not address the topic of interest for the study: 10 were excluded during the full-text reading stage because of full text unavailability and 83 were excluded because of the type of study, resulting in the inclusion of 79 studies for full analysis. The search and article selection flowchart is presented in Figure 1.
Out of the 79 included studies, 50.63% were case reports, 18.99% were case series, and 30.38% were analytical studies, the majority of which were cross-sectional studies (n=20), followed by cohort studies (n=2), and case-control studies (n=2). The most frequently reported COVID-19 - associated neurological manifestation was impaired sense of smell and/or taste (43.04%), followed by peripheral neuropathy (20.25%), seizures (8.86%), encephalitis (7.59%), and delirium (5.06%). Other manifestations less frequently described were headache, myositis, stroke, and transverse myelitis (Table 1). In addition, most of the studies were published in the United States (24.05), Italy (13.92%), and Spain and the United Kingdom (8.86% each).
Table 1 Distribution of the most common neurological manifestations reported in COVID-19 patients by publication type.
| Publication type | |||||||
|---|---|---|---|---|---|---|---|
| Case report (individual) (n=40) | Case series (n=15) | Cross-sectional study (n=20) | Cohort study (n=2) | Case-control studies (n=2) | Total | ||
| Smell and/or taste impairment | 8 | 6 | 18 | 0 | 2 | 34 | |
| Neurological manifestation | Peripheral neuropathy | 11 | 5 | 0 | 0 | 0 | 16 |
| Seizures | 5 | 2 | 0 | 0 | 0 | 7 | |
| Encephalitis | 6 | 0 | 0 | 0 | 0 | 6 | |
| Delirium | 2 | 1 | 0 | 1 | 0 | 4 | |
| Other * | 8 | 1 | 2 | 1 | 0 | 12 | |
* Case report consisting of three cases with stroke; series of two cases of vasovagal syncope; two individual case reports of rhabdomyolysis; two individual case reports of visual impairment; an individual case report of ataxia, headache, and decreased consciousness.
Source: Own elaboration.
Demographic characteristics, presence of comorbidities, and COVID-19 severity according to neurological manifestation described in individual case reports and case series are presented in Table 2.
Table 2 Summary of individual cases 6,12-50and case series.51-65
* Other: case report of three stroke cases; series of two cases of vasovagal syncope; two individual case reports of rhabdomyolysis; two individual case reports of visual impairment; an individual case report of ataxia, headache, and decreased consciousness. wIQR: interquartile range.
Source: Own elaboration.
In addition, Table 3 presents the objective, sample, population characteristics, severity of SARS-CoV-2 infection, primary and secondary neurological manifestations, and findings of all analytical studies (descriptive, cross-sectional, case-control, and cohort) on neurological manifestations in patients with COVID-19.
Table 3 Summary of descriptive, cross-sectional, case-control, and cohort studies on neurological manifestations in COVID-19 patients.66-89
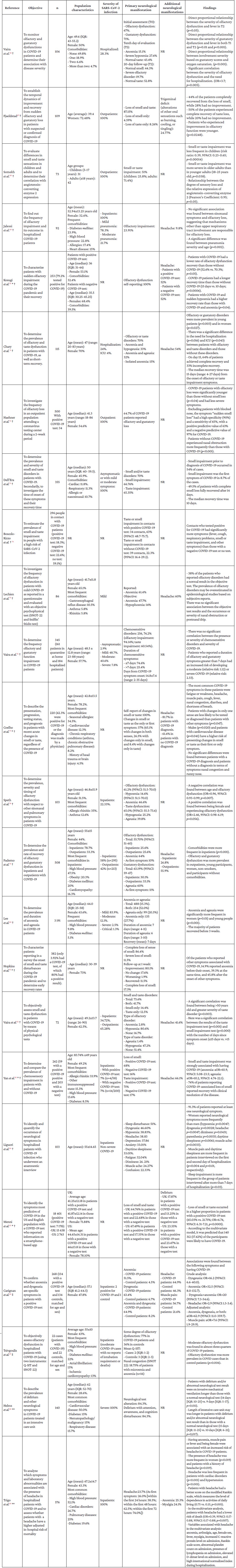
aOR: adjusted odds ratio; CI: confidence interval; IQR: interquartile range; NR: not reported; OR: odds ratio; Q-SIT: Quick Smell Identification Test; SNOT-22: SinoNasal Outcome Test 22; UK: United Kingdom; US: United States of America.
* Data collection via internet or mobile applications.
† Cross-sectional study.
‡ Case-control study.
** Cohort study.
Source: Own elaboration.
Discussion
The articles retrieved describe the neurological manifestations that were associated with COVID-19 during a 6-month period following the WHO declaration of the pandemic.2 The main findings of these studies are presented below by type of neurological manifestation.
Impaired sense of smell and taste
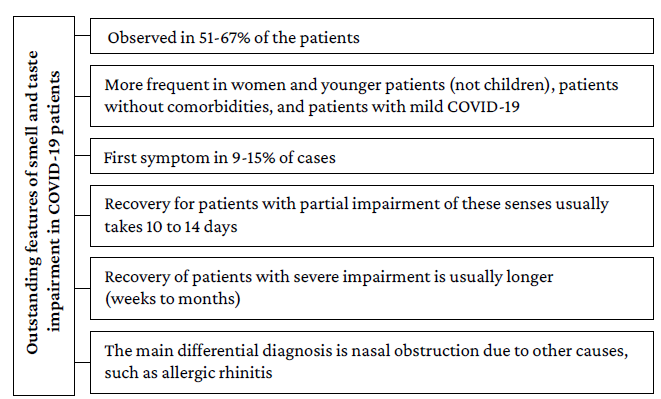
Most of the publications included in the present review report an impaired sense of smell and taste (43%). Moreover, evidence suggests that between 51% and 67% of patients with mild COVID-19 report impairment of these senses. However, only one study80 conducted in South Korea in 3 191 patients reported the prevalence of acute anosmia or ageusia in 15.3% (n=488/3 191) of patients in the early stages of COVID-19 and 15.7% (n=367/2 342) of patients with asymptomatic-to-mild disease severity. It should be noted that the main limitation of this research is that it studied the complete impairment of these senses, omitting the review of partial alterations.
On the other hand, a lower prevalence of these disorders was observed in patients with moderate and severe COVID-19 (23%-40%, respectively), since most of the analytical studies reviewed reported that the presence of these neurological manifestations was significantly more frequent in young people, women, patients without comorbidities, and patients with mild COVID-19. Furthermore, the only publication suggesting that there might be a direct association between disease severity and olfactory impairment is the study by Vaira et al.,66 in which a statistically significant correlation was found between the severity of olfactory or gustatory impairment and the presence of fever, altered oxygen saturation, and requirement for hospitalization in patients with severe SARS-CoV-2 infection.
Studies using standardized tools for measuring smell and taste impairment (such as the SinoNasal Outcome Test 22 [SNOT-22] and the Quick Smell Identification Test [Q-SIT]) confirm that COVID-19 patients experience these impairments more frequently than the general population.75,82,87 Likewise, these studies suggest that the duration of sensory impairment is proportional to its severity. These papers also report that smell and taste disorders cannot be objectively confirmed in 26-51% of the patients who report them.
Regarding the usefulness of smell and taste impairment as a marker for COVID-19, three aspects stand out. First, case-control studies clearly showed that these disorders are significantly more frequent in patients with COVID-19 than in the general population.86,87 Second, individuals who report the presence of smell and taste impairment are more likely to have COVID-19 if they also have other symptoms of infection; for example, Menni et al.85 report that smell and taste impairment, fatigue, persistent cough, and loss of appetite were significantly associated with SARS-CoV-2 infection. Thirdly, it has been reported that the main differential diagnosis in cases of sudden smell and taste impairment is nasal obstruction due to other causes, such as allergic rhinitis.72
Concerning the onset and duration of symptoms, data are quite heterogeneous. The reviewed publications report that between 9% and 15% of patients presented anosmia as the first symptom of the disease.73,81 Also, most patients report partial smell and taste impairment and a recovery time of 10 to 14 days, although some cases may require up to 1 month to recover.71,73,80
It is noteworthy that recovery seems to be related to the degree of smell and taste disorder, since patients with anosmia took longer to recover than those with hyposmia.66,70,76,82
Likewise, a prolonged duration of these impairments could be associated with the severity of COVID-19; however, the correlation between the severity of smell and taste impairment and the severity of COVID-19 has not been clearly established.66 The most relevant aspects of this disorder are presented in Figure 2.
Peripheral nerve involvement
Sixteen articles (case studies and case series) were retrieved, reporting 20 COVID-19 patients with neuropathies, polyneuropathies, and polyradiculopathies. In general, these patients were younger than 60 years of age (only 7 were older) and had few comorbidities since only three of them had hypertension and one had paranoid schizophrenia (a 48-year-old man). Additionally, more than half of these patients had mild COVID-19, while only 3 had severe COVID-19, and no participant died. This may be related to the moment of neuropathy onset because, in most cases, the neuropathy occurred after the acute stage of the disease.
Regarding the type of neuropathy, most cases corresponded to Guillain-Barré syndrome (GBS) or one of its variants, and its onset occurred between 6 and 21 days after the onset of signs and symptoms; it was also reported that these neurological manifestations usually occurred after the onset of respiratory and systemic symptoms of COVID-19. This is consistent with reports in similar studies.90
Other types of neuropathy found were facial paralysis, hearing impairment, and supranuclear ophthalmoplegia. In general, the prognosis of patients with peripheral nerve involvement was good and chronic symptoms were reported in only one case. This case involved a 72-year-old man who had had mild COVID-19 (which manifested only with diarrhea) and whose comorbidities included hypertension, coronary artery disease, and alcoholism. This patient presented a very severe form of GBS and dysautonomia and had an unfavorable progression, requiring tracheostomy and percutaneous endoscopic gastrostomy tube placement.28
Headache
While only one of the retrieved analytical articles focuses on investigating the association between headache and COVID-19, 11 publications report data on this symptom (Table 3).69-71,75,77,79,82-84,86,89 In 8 of them, the prevalence of headache in patients with COVID-19 exceeds 50% and even reaches 82%. Importantly, most of these patients had mild SARS-CoV-2 infection. In fact, prevalence data below 50% is for patients with moderate and severe disease.
Despite the above, headache is considered a nonspecific symptom and is not included in the items considered in the diagnostic algorithms for COVID-19. In general terms, this symptom seems to be more frequent in women and in patients with a history of primary headache.89
Distribution of neurological manifestations at different age ranges
The behavior of neurological manifestations in patients with SARS-CoV-2 infection had a different distribution across extremes of age. One of the conditions presenting in elderly patients is delirium. In the case reports and case series in which this disorder was reported,30,31,57,62 all patients (n=8) were older than 65 years and, at the same time, they had more comorbidities than the patients described in the other case reports included in the present review. In addition, half of these patients had mild COVID-19 (n=4), but it is striking that 3 of these participants had dementia and the other had paranoid schizophrenia. These findings confirm that patients with dementia, even if they have mild COVID-19, are at increased risk of developing delirium. Moreover, case reports reporting data on symptoms of delirium or mental confusion indicate that these symptoms appeared within the first four days from the onset of disease symptoms,31,62 suggesting that delirium may be an early neurological manifestation in older adults.
On the other hand, it was found that the presence of delirium is a marker for poor prognosis in older adults with severe COVID-19. In this regard, in the study by Helms et al.,88 patients with delirium required invasive mechanical ventilation for a longer time and had a longer stay in the intensive care unit (ICU) than those without delirium and with a normal neurological examination.
At the other extreme of age, very few articles reported data on pediatric patients, which is a limitation for the representativeness of all population groups. On this point, only four of the publications reviewed explicitly refer to children; actually, being of legal age is one of the inclusion criteria in most of the studies reviewed. In particular, two of the four articles report smell and taste impairment. On the one hand, in the study by Somekh et al.,68 in which 31 of the patients were between 5 and 17 years of age, it was found that these symptoms were less frequent in this age group (children: 26%; adults: 71%). On the other hand, in the United Kingdom, Mak et al.,55 described three cases of adolescents between 14 and 17 years of age with COVID-19 who presented loss of smell and/or taste.
The other two articles are individual case reports. The first reports the case of a previously healthy 11-year-old boy who presented status epilepticus and was subsequently diagnosed with encephalitis based on the findings of cerebrospinal fluid analysis. Based on what was reported, the patient tested positive for COVID-19 as well as for rhinovirus/ enterovirus on nasal swab test, although no evidence of the latter was found in this fluid, and finally, the child recovered completely in 6 days without the need for treatment.41
The second reports a case of COVID-19-associated rhabdomyolysis in a 16-year-old patient with autism spectrum disorder, attention deficit hyperactivity disorder, morbid obesity, obstructive sleep apnea, and eczema. According to this report, the patient, who tested positive for COVID-19, presented with myalgia, fever, mild shortness of breath, and dark-colored urine, and his creatine phosphokinase level was 427 656U/L, leading to a diagnosis of rhabdomyolysis. Finally, the child progressed satisfactorily during hospitalization and was discharged after 12 days with creatine phosphokinase levels of 6526U/L.45
Severe neurological manifestations
In addition to severe forms of GBS and cases of delirium that were associated with extubation difficulties and longer ICU stay, other severe neurological manifestations associated with COVID-19 have been described. Although the case of a pediatric patient with encephalitis was mentioned above, inflammatory involvement of the central nervous system was also reported in five other patients in the publications reviewed. Of these, a case of acute disseminated encephalomyelitis following SARS-CoV-2 infection in a 64-year-old woman with hypertension and vitiligo stands out.42 Also, 3 patients between 35 and 59 years of age (2 with severe COVID-19 and 1 with mild COVID-19) were reported to have encephalitis in the second week after symptom onset.37-39 It is noteworthy that only 1 of these 3 patients had comorbidities and, in fact, was the only one who died.
Likewise, a series of three cases of patients aged 33, 77 and 55 years with mild COVID-19 from the United States, who were admitted to the emergency department due to clinical symptoms of stroke, was retrieved. In these three cases, internal carotid artery thrombosis was documented.60
In addition, epileptic seizures associated with SARS-CoV-2 infection were reported in 6 patients, of whom 1 had severe infection and 2 had a previous diagnosis of epilepsy.32,33,35,36,64 In 5 of these patients, the onset of seizures was focal, while in the other one it was described as generalized tonic-clonic. In turn, 4 of the 6 patients presented status epilepticus, of which 2 had de novo status epilepticus, 1 had sequelae of herpetic encephalitis, and the other had thrombosis of the venous sinuses requiring craniotomy. Besides epileptiform activity, electroencephalogram monitoring reported encephalopathic tracing and graphoelements, such as periodic lateralized epileptiform discharges.
Other non-epileptic paroxysmal disorders were also described in some patients. The occurrence of vasovagal syncope was reported in 5 patients, most of whom were over 65 years of age,34,65and only 1 of them reported the presence of convulsive epileptic seizures associated with vasovagal syncope, as well as autonomic dysfunction.34 Finally, a series of three patients with generalized non-epileptic myoclonus, similar to the startle reflex, was interpreted as a para-infectious immune-mediated disorder.63 None of the patients with vasovagal syncope or myoclonus had severe COVID-19.
Regarding the limitations of this literature review, two aspects should be mentioned that could have affected the inclusion of all the articles published on this topic in the period analyzed.
First, there is an underreporting of clinical manifestations that may be interpreted as general manifestations, but which, in other contexts, may be considered neurological. Such is the case of symptoms like myalgias, which are not the main objective of any of the articles retrieved, but are reported in at least 12 of the patients described, of which only 3 presented rhabdomyolysis.16,17,23,35,37,44-47,50,63,64 In addition, some of the descriptive articles reviewed report prevalence figures of myalgia in patients with COVID-19 between 25% and 57%,84,86,89 which is similar to those described in patients with headache, as mentioned above.
Second, some articles may not have been identified because, although they address this topic, they do not meet the parameters of the proposed search strategy, for example, those that do not include the selected keywords. Despite the above, the results obtained in this literature review allow us to establish an overview of the neurological manifestations in patients with COVID-19 infection.
Conclusions
In the six months following the declaration of the COVID-19 pandemic, multiple articles were published reporting data on the spectrum of neurological manifestations associated with SARS-CoV-2 infection. The search, review and analysis of these studies provided a general overview and led to the conclusion that, although the main involvement of COVID-19 is pulmonary, the nervous system can also be affected directly or indirectly. While taste and smell impairment is the most studied and reported neurological manifestation, a wide number of such manifestations were found to be associated with the disease.
Some of these neurological manifestations, such as headache and myalgia, are underreported because they are considered general symptoms and signs of the disease. However, other more specific manifestations such as epileptic seizures, peripheral nerve inflammation, encephalitis, delirium, and stroke have been described. Most of them do not seem to be related to the patient's previous health condition or to the severity of COVID-19. Furthermore, other neurological manifestations such as delirium and epileptic seizures may be more frequent in patients with a history of dementia and epilepsy, respectively.
Finally, it has been reported that the prognosis of patients with these neurological manifestations is usually favorable, except in those with conditions that cause sequelae, such as GBS or stroke. Future research is expected to better characterize the mechanisms underlying nervous system involvement in patients with COVID-19.