INTRODUCTION
Nitrification plays an important role in the global nitrogen (N) cycle in most agroecosystems. Ammonium oxidizing bacteria (AOB) are mostly responsible for the transformation of ammonium to nitrate, via nitrite, which is limited in agricultural land by practices that degrade soil health and functions (Prosser and Embley, 2002). It is known that terrestrial AOBs belong to a monophyletic group within the Proteobacteria sub-class, and the currently accepted classification recognizes only two genera within this group, Nitrosospira and Nitrosomonas (Stephen et al., 1996).
The quantification of these bacteria through the amoA genes is valuable, because N is the most important nutrient in the agroecosystem, given its limitation in edaphic conditions and high participation in multiple biochemical reactions physiologically involved in the growth, development and production of crops (Rao, 2009).
N is crucial in the productivity of cultivars such as soy (Glycine max L.) and corn (Zea mays L.), which are basic in the human and animal diet. Nitrogen fertilization in production costs ranges from 15 to 30%. Therefore, it is one of the most expensive elements to supply through industrial synthetic inputs (FENALCE, 2011). The main sources of N for crops are the mineralization of organic N from the soil and the addition of synthetic and organic fertilizers. Much of the total N (95%) supply to the soil is due to the presence and mineralization of organic matter (Philippot and Germon, 2005).
The most used way to contribute N to crops is by synthetic route. FAO (2017) states that worldwide, in 2020 about 119 thousand tons of nitrogen fertilizers will be used, where the efficiency in agroecosystems does not exceed 33% (Glass, 2003). The low efficiency of N is typically caused by agronomic practices such as excessive fertilization, compaction and gravity irrigation, promoting N losses through routes such as erosion, leaching and volatilization.
Given the accelerated degradation of soils, it is necessary to recover, conserve and increase their fertility in a healthy and lasting way. One of the technologies applied in the process is F, a conventional system that provides biomass of native species that grow postharvest. Another alternative of agro ecological cut is the sowing and incorporation of Mucuna pruriens L. as green manure (GM) or arranged on the ground as organic mulch (OM), practices used in association or in rotation that provide plant material in situ to maintain, improve or restore the physical, chemical and biological properties of the soil (Zribi et al., 2011).
These materials increase the efficiency in the use of N through symbiotic relationships such as the biological fixation of N in legumes (Shoko et al., 2007) and nutrient recycling by stimulating the growth of soil biota, which contributes considerable amounts of organic matter, improves the microbial action on the substrate and makes available to the attached or subsequent crops, nutrients and water (Baijukya et al., 2004). The objective was to characterize the population changes of the AOB, the availability of NO3 - and the yield of corn and soybeans under the use and management of GM, OM and B, together with nitrogen fertilization.
MATERIALS AND METHODS
Characterization of the study area. The work was carried out in the Experimental Field of the National University of Colombia, Palmira headquarters (CEUNP), Valle del Cauca, Colombia. This field is located at coordinates 3°25'34'' LN and 76°25'53'' LO, at an altitude of 980 masl, e temperature of 24°C, relative humidity of 69% and annual rainfall of 1406mm.
The soil corresponded to a typical isohyperthermic thin frankish Haplustert with a 1% slope, which has been in F, approximately eight years, which is characterized chemically (Table 1) and physically from the soil seven days before installing the test. For the interpretation of chemical properties, criteria managed at the national level were used (Castro, 2004). This soil has neutral pH, medium concentration of inorganic CO and N, high level of phosphorus and sulfur. For the bases, average levels of Na+ and K+ and high levels of Mg2+, Ca2+ and CEC were found. For the minor element B, the level was high, and very high for Mn; low for Cu and Zn and very low for Fe, which are shown as limiting.
Table 1. Initial chemical characterization of a Typic Haplustert at a depth of 0 - 20cm, used to evaluate the effect of green manures on the activity of nitrifying bacteria
| CO | pHH2O | N Total | NH4 + | NO3 - | P (Bray II) | S | B | Fe | Mn | Cu | Zn | K | Ca | Mg | Na | CEC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| g kg-1 | -------------------------------------------mg kg-1-------------------------------------- | --------cmolc kg-1---------- | ||||||||||||||
| 26.6 | 6.84 | 1.174.5 | 4.42 | 13.84 | 57.45 | 28.03 | 1.45 | 8.83 | 61.75 | 0.67 | 1.49 | 0.44 | 10.9 | 5.63 | 0.10 | 21.10 |
The analysis of the physical properties indicated a loamy clay texture, which allows adequate workability as long as the gravimetric humidity is not high (Jaramillo, 2002). The heavy texture plus the high apparent density found (1.72g cm3), which is reflected in very low total porosity (35.34%), can generate conditions of reduction or impediment to the root development of crops.
Treatments and experimental design. To meet the proposed objectives, treatments were designed under the design of Random Complete Blocks with 32+2 factorial arrangements with three repetitions (Table 2). In the first phase of research, the plant component (GM / OM or F) was established and managed prior to the establishment of the crops; followed, the associated crop (corn + soy) and monoculture (corn or soy) systems were sown. Each plot was subject to different fertilization program (compost, synthetic fertilizer and no addition).
Table 2. Description of treatments used to evaluate the effect of green manures on the activity of nitrifying bacteria.
| Treatment | Symbol |
| GM (Mucuna pruriens & Zea mays L.) + intercropping corn and soy + organic fertilization | T1 (GM-OF) |
| GM (Mucuna pruriens & Zea mays L.) + intercropping corn and soybean + synthetic fertilization | T2 (GM-CF) |
| GM (Mucuna pruriens & Zea mays L.) + intercropping of corn and soy + without fertilization | T3 (GM-NF) |
| OM (Mucuna pruriens & Zea mays L.) + intercropping corn and soy + organic fertilization | T4 (OM-OF) |
| OM (Mucuna pruriens & Zea mays L.) + intercropping corn and soybean + synthetic fertilization | T5 (OM-CF) |
| OM (Mucuna pruriens & Zea mays L.) + intercropping of corn and soy + without fertilization | T6 (OM-NF) |
| F (Rottboellia cochinchinensis L.) + intercropping corn and soy + organic fertilization | T7 (F-OF) |
| F (Rottboellia cochinchinensis L.) + intercropping corn and soybean + synthetic fertilization | T8 (F-CF) |
| F(Rottboellia cochinchinensis L.) + intercropping of corn and soy + without fertilization | T9 (F-NF) |
| F (Rottboellia cochinchinensis L.) + monoculture corn + synthetic fertilization | T10 (F-CF) |
| F (Rottboellia cochinchinensis L.) + monoculture soy + synthetic fertilization | T11 (F-CF) |
Agronomic management of the plots. The plots were prepared with two landfill plow passes, with a dimension of 20m2 (6 x3.34m) for treatments from one to nine and 30m2 (6 x 5m) for treatments 10 and 11. Subsequently, a rain gauge and thermometer in order to estimate the irrigation that fell on the ground (rainfall + irrigation) and the ambient temperature of the area during the development of the experiment (Figure 1).
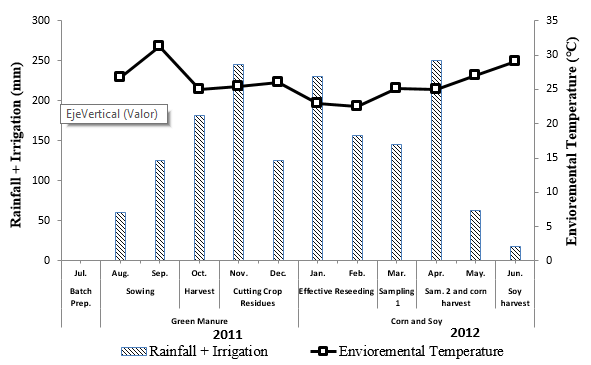
Figure 1. Monthly distribution of the environmental temperature and sheet of water in the soil (rainfall + irrigation) during the experiment.
After plotting the experimental units, the legume M. pruriens L. var. utilis (CIAT No. 9349) and Z. corn (variety ICA 305) in the experimental units corresponding to treatments one to six. They were allowed to grow for three to four months until the reproductive stage (R3). After the harvest of corn, the biomass (addition or incorporation) of the GM was cut, chopped and allowed to decompose in the first five centimeters of the soil for 30 days. The F management in the plots of the rest of treatments (T7 to T11), was cut and left on the soil surface.
The establishment of the corn crop variety ICA 305 was sown at a density of 40,000 seeds ha-1, while the soybean variety ICA P34 was 200,000 seeds ha-1 , with a ratio of one furrow of corn for two soybeans in each experimental unit.
Fertilizers with organic and synthetic sources were performed in bands located at the foot of the plants, and at 15 and 45 days after the effective planting of crops. Synthetic fertilization was performed by applying the triple fifteen fertilizer (15-15-15) at a dose of 50kg ha-1 of N, P and K (335g per application on 20m2 plots with interculture and 501g for the plots of 30m2 with monoculture) (Moreno et al., 2008). For treatments with the addition of compost, the dose was calculated to provide the same amount of N as the synthetic fertilizer; in that sense, this consisted of dried and composted chicken manure in doses of 3.4t ha-1 (3.4kg / application), which is equivalent to 50kg ha-1 of N. The other treatments were managed without the application of fertilizers or amendments.
Response Variables. To know the activity of nitrifying bacteria, in the corn and soybean system, the variables related to soil, such as ammonium oxidizing bacteria, NO3 - were analyzed by spectrophotometer colorimetry in KCL 1M (Borrero et al., 2017), volumetric humidity (Jaramillo, 2002) and temperature. At each stage of flowering and grain filling, nine soil subsamples and three leaves / plant were located opposite the spike of the 10 corn plants sampled. In the stage of physiological maturity, the harvest was collected and the yield of corn and soybean crops was determined.
For the absolute quantification of the amoA genes of the soil AOB, we proceeded according to the protocol developed and standardized by CIAT (Moreta, 2009). The DNA from the soil samples was extracted using the FastDNA® SPIN kit (Biomedicals MP, Cat. No. 116560200), then quantified by fluorescence with the PicoGrenn® dsDNA reagent (Laboratory: Molecular Probes) and subsequently subjected to electrophoresis in a 1% agarose gel to determine its quality. The estimation of the number of copies of the amoA gene of the BOA was done through real-time PCR (qPCR) using the pair of primers (sense 5'-3 '): amoA - 1F GGGGTTTCTACTGGTGGT and amoA - 2R CCCCTCKGSAAAGCCTTCTTC, whose product PCR is 493 base pairs (bp) (Rotthauwe et al., 1997).
The genes under study were quantified using the Brilliant II SYBR® Green QPCR Master Mix fluorescent dye (Agilent Technologies, Cat. No. 600828). The qPCR reactions were run in triplicate in a 20μl volume per reaction, containing 20ng of soil DNA, 0.5μM of each first and 10μl of Brilliant SYBR Green qPCR Master Mix. The negative control consisted of water instead of DNA and, as a positive control, plasmid DNA was used which contained the amoA gene insert. The reactions were run on an OPTICONTM2 thermocycler (Continuous Fluorescence Detector) (MJ Research) and analyzed with the MJ OPTICONTM Software version 3 (BIO-RAD). The amplification conditions of the gene of interest were the following: (i) 95°C, 5min; (ii) 95°C, 1.5min; (iii) 55°C, 1.5min; (iv) 72°C, 1.5min. The raw data (number of gene / reaction copies) were corrected using soil gravimetric moisture and then expressed as number of copies of gene / g dry soil.
Analysis of the information. The information obtained was submitted to the Analysis of Variance (p˂0.05), Duncan averages test (p˂0.05), orthogonal contrasts and pearson correlations through the use of SAS software version 9.1.3 (SAS, 2002).
RESULTS AND DISCUSSION
During flowering, significant differences (P <0.05) were found between treatments for NO3 -temperature and volumetric humidity, but not in ammonia-oxidizing bacteria (AOB) populations, where the total rainfall during the investigation stage was 145 mm. The NO3 - concentrations were not statistically different, with the exception of GM-NF, where the lowest value was found (Figure 2). Although the AOB populations did not differ statistically, the same trend was found as Abbott and Murphy (2007), who found that the populations are higher when CF is applied, compared with OF and NF. In this investigation, it was confirmed that the nitrification intermediaries, AOB and NO3 -, are considerably affected by environmental and soil conditions, especially humidity (Brady and Weil, 2004; Gallego, 2012).
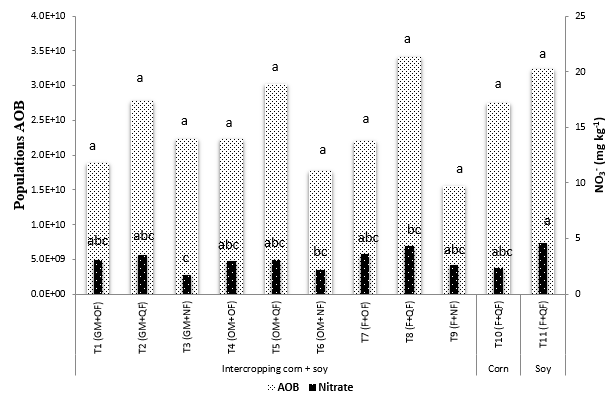
Averages with the same letters are not significantly different according to Duncan (P <0.05).
Figura 2. AOB (number copies amoA / g dry soil) and NO3- (mg kg-1) in the treatments evaluated during the flowering stage of the crops.
As for the soil temperature (Figure 3), it differed significantly between treatments, in the corn monoculture the highest soil temperature (24.97°C) was found while the lowest (24.29°C) where the GMs were incorporated. With respect to volumetric humidity, in the plots where the high quality organic materials were incorporated (GM) or on the ground (OM) was combined with OF stored the highest humidity (26.43%), although it did not differ statistically with the other management systems. The lowest humidity values without statistically differing, were found under the combination OM-NF and B-CF. Zribi et al., (2011) reported that these variables fluctuate in agricultural soils in Spain, due to the addition and use of GM and OM, since during the mineralization the microbial, nutritional and water circulation activity is energized, all of this coupled with the edaphoclimatic variability.
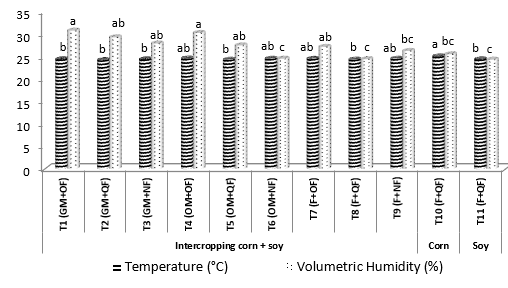
Averages with the same letters are not significantly different according to Duncan (P <0.05).
Figure 3. Temperature (°C) and Volumetric Humidity (%) in the treatments evaluated during the flowering stage of the crops.
In the grain filling stage, significant differences (P <0.05) were found between treatments for temperature and volumetric humidity in the soil, but not for the AOB and NO3 - populations (Figure 4). Compared to flowering, the populations of nitrifying bacteria decreased by 15%, and as a result, the nitrate concentration increased, this process was affected by the rainfall. Even so, the population averages found in this trial, using the qPCR were higher (oscillate between 2.5E+9 and 3.4E+10) than the records in other edaphic media, in which populations were between 9.5E+4 and 2.0E+7 copies of amoA g-1 soil gene (Moreta et al., 2009; Szukics et al., 2010; Cao et al., 2011).
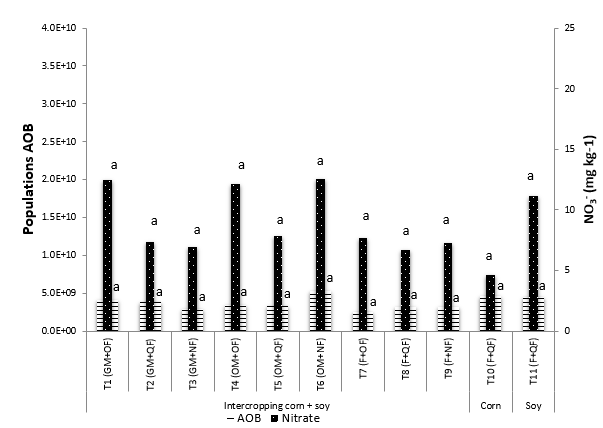
Averages with the same letters are not significantly different according to Duncan (P <0.05).
Figura 4. AOB (number copies gene amoA / g dry soil) and NO3 - (mg kg-1) in the treatments evaluated during the grain filling stage of the crops.
In this work it has been shown that GM and OM constitute better nitrogen sources compared to the F, given the greater contribution of organic matter and its C:N ratio, which makes them rapidly metabolizable. The variables that underwent major changes in the trial were soil temperature and humidity. In spite of the evidences that the statistical analyzes carried out, when Pearson's correlation was performed in order to corroborate the influence of some variables evaluated with the activity of the AOB, the results were not significant, which can be explained by the high coefficients of variation, especially soil moisture, given that the test was carried out in field conditions, where the soil is variable due to its genesis environment and management (Malagón et al., 1995).
Several authors agree on the high influence of aerobic conditions on AOB populations and therefore on the production of NO3 - (Stres et al., 2008; Montaño et al., 2013). It is scientifically proven that the process of ammonification occurs through aerobic and anaerobic pathways, while nitrification is a purely aerobic process (Orozco, 1999). Stres et al. (2008) have also found that temperature also has a high influence on the activity of these populations. They affirm that if the temperature reaches 25 to 30°C, AOB activity is maximized, which is consistent with t this investigation (the temperature ranged between 25.5 and 29.3°C).
In the analysis of soil temperature (Figure 5), statistical differences between treatments were recorded. The lowest values (25.37°C) were presented in the soil management with GM. The highest volumetric humidity was found with the application of GM plus CF (25.79%) and OM combined with OF (24%), while in the F in all conditions, the lowest record was found with an average of 19%.
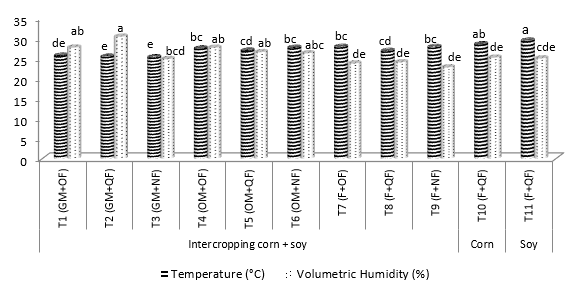
Averages with the same letters are not significantly different according to Duncan (P <0.05).
Figure 5. Temperature (°C) and Volumetric Humidity (%) in the treatments evaluated during the grain filling stage of the crops.
Several investigations have shown that soil moisture and temperature affect nitrification. Authors such as Rodríguez et al. (2007) and Szukics et al. (2010) have confirmed a directly proportional trend between the temperature and the activity of nitrifying bacteria when aerobic conditions predominate. With this information, it is likely that in the stage of grain filling, where there was an increase in the temperature in the soil, the highest rainfall (250mm) referenced, have affected the activity of the nitrifiers and therefore, the production of NO3 -.
The analysis of orthogonal contrasts related to the activity of AOB (Table 3), confirmed that the populations were similar between treatments compared, as were the concentrations of NO3 - in intercalated crops. It was found that the application of GM significantly favored greater volumetric humidity compared to the F in the flowering stage and, in grain filling, both GM and OM favored this variable with respect to the F. At the time of mineralization of 4 t ha 1 of the organic materials deposited as GM or OM, positively affected through greater moisture and nutrients to the system. The soil temperature was significantly increased under the conditions OM (2.1ºC) and the F (2.2ºC). These results confirm the trends observed in the previous statistical analyzes.
Table 3. Orthogonal contrasts for AOB, NO3 - and physical soil variables related to nitrification in the phenological stages evaluated.
| Flowering stage | |||||
|---|---|---|---|---|---|
| Source of variation | AOB | NO3 - | Volumetric humidity | Soil Temperature | |
| Genes copies number amoA/g dry soil | mg kg-1 | % | °C | ||
| GM vrs OM | ns | ns | ns | ns | |
| GM vrs F | ns | ns | * | ns | |
| OM vrs F | ns | ns | ns | ns | |
| OF-NF vrs CF in GM | ns | ns | ns | ns | |
| OF-NF vrs CF in OM | ns | ns | ns | ns | |
| OF-NF vrs CF in F | ns | ns | ns | ns | |
| Grain filling | |||||
| GM vrs OM | ns | ns | ns | * | |
| GM vrs F | ns | ns | * | * | |
| OM vrs F | ns | ns | * | ns | |
| OF-NF vrs CF in GM | ns | ns | * | ns | |
| OF-NF vrs CF in OM | ns | ns | ns | ns | |
| OF-NF vrs CF in F | ns | ns | ns | ns | |
1: GM= Green manure; OM= Organic mulch; F= fallow; OF= Organic fertilization; CF= Chemical fertilization; SF= No fertilization* = there is a significant difference, ns = not significant.
Regarding crop yield, no significant differences were found between treatments applied to corn (Figure 6). The values ranged between 4.2 t ha-1 and 6.0 t ha-1, higher than the average recorded in traditional systems (1.9 units) and similar to those technified (5.0units) in Colombian agriculture (FENALCE, 2011). Similar results were found in experiments conducted in the Coffee Zone and Atlantic Coast, where production with improved materials and synthetic fertilization ranged between 4.6 t ha-1 and 4.8 t ha-1 (Granada et al., 2008; Criollo et al., 2013).
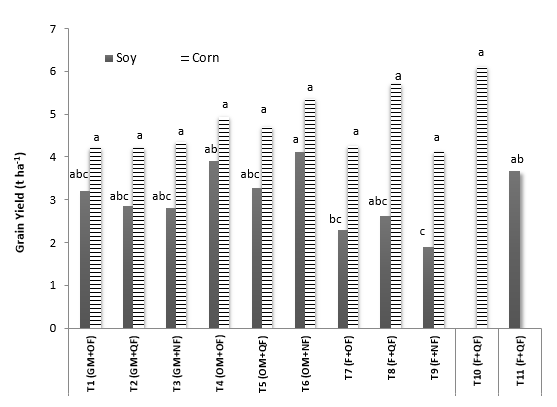
Averages with the same letters are not significantly different according to Duncan (P <0.05).
Figure 6. Grain yield of corn and soybean crops in the evaluated treatments.
The yield in soybeans presented significant differences (P <0.05) between treatments (Figure 6). In interleaved crops (GM, OM and F) yield values were statistically similar, with the exception of F-NF, where the lowest value (1.9 t ha-1) was recorded, while the highest was in OM-NF (4.11 t ha-1), which was similar to the yield found in soybean monoculture. Most of the yields observed in this investigation (1.9 to 4.1 t ha-1) were higher than those reported in other works that used this variety, which ranged between 1.66 t ha-1 and 2.11 t ha-1 (FENALCE, 2011; Valencia-Ramirez and Ligarreto-Moreno, 2012). Constant humidity and nutrient availability in the soil, plus management conditions facilitated a good phenotypic expression of the genetic material evaluated, as reported in conditions of Ecuador (Campoverde and Cabrera, 2006) and Argentina (Boga and Ramírez, 2014).
CONCLUSIONS
The populations of ammonium oxidizing bacteria (AOB) and their metabolic product NO3 - did not vary significantly between treatments. Its expression was influenced by edaphoclimatic conditions, especially soil moisture.
In the intercropping corn-soybean, the use of organic materials such as green manure / organic mulch promoted in corn similar yields to those obtained with synthetic fertilizers, while in soybean when organic padding was added the yields exceeded the average of the traditional fallow system.














