INTRODUCTION
High Andean forests are strategic ecosystems with high diversity of native and endemic plants. These forests regulate water interception, store carbon, and increase soil fertility (Mancipe-Murillo et al., 2018; Melissa and Torres, 2016; Quintero et al., 2017). In Colombia, this natural ecosystem covers 24.9% of its surface; however, natural and anthropic factors, such as land use and land use change (LULUFC) exert high pressure on this ecosystem (Cárdenas-Burgos et al., 2019; Quintero et al., 2017).
One of the challenges of ecological restoration is the recovery of this forest ecosystem using native pioneer species that favor the establishment of ombrophytes and contribute to the exchange of soil nutrients (Acero-Nitola and Cortés-Pérez, 2014). Therefore, knowledge of the main soil physical factors and their relationship with water and temperature is essential to efficiently propagate these species (Sánchez et al., 2019). Consequently, the propagation of wild plants, in nurseries using low-cost pre-germination techniques, may constitute a sustainable management method (Aguilar and Vanegas, 2009; Espitia et al., 2016; Sánchez et al., 2015).
The tree species: Viburnum triphyllum Kunt. (Caprifoliaceae), Oreopanax floribundum Kunth. (Araliaceae), Weinmannia tomentosa L.f. (Cunoniaceae), and Tournefortia fuliginosa Benth. (Boraginaceae), commonly known as pelotillo, mano de oso, encenillo, and pundé, respectively, are relevant components for the diversity of the High Andean ecosystem. These native species are valuable for restoration and revegetation processes in forests and water sources due to their morphological, physiological, and ecological characters and cultural importance (Bacca and Burbano, 2018; Hernández-Pineda et al., 2014).
Despite the value of wild species, studies on the germination of native High Andean species are currently scarce (Mancipe-Murillo et al., 2018). Moreover, there is a little knowledge of the effectiveness of pre-germination treatments for plant propagation in restoration projects (Dayrell et al., 2016; Di Sacco et al., 2018; Espitia et al., 2017; Sánchez and Furrazola, 2018; Sánchez et al., 2018a). However, recent researches on native species have been addressed, for instance, the performance of V. triphyllum seedlings under two treatments in pasture zones, reporting higher survival rates in the treatment without nutrient amendment. This finding demonstrates the establishment of seedlings in degraded sites and eroded soils and reduces nursery costs (Hernández-Pineda et al., 2014). Furthermore, a study on carbon capture of O. floribundum was conducted for restoration efforts in La Poma ecological park in Bogotá. The species showed fast growth and capacity to overcome water deficit, since 30% of the biomass carbon content was present in the root system (Díaz and Velásquez, 2015). Moreover, Bacca and Burbano (2018) assessed the growth of O. floribundum seedlings, concluding that this species is suitable for ecological restoration since it grows in areas with agricultural and livestock impacts. In a nursery, Aguilar and Vanegas (2009) evaluated the germination of different native species to generate a source of plant material and baseline knowledge. Among the species studied, V. triphyllum, O. floribundum, and W. tomentosa are timber trees used to promote forest restoration, protect water sources and soil quality. Furthermore, propagation of these species is mainly done through the use of seeds, which must be subjected to pre-germination treatments through imbibition for different periods and at variable temperatures. Finally, T. fuliginosa, a fast-growth tree, is use to basin’s river protection (Toro, 2010).
On this basis, this study evaluated the germination of O. floribundum, W. tomentosa, T. fuliginosa, and V. triphyllum with different substrates and pre-germination managements in the nursery of Botana experimental farm to contribute knowledge of the propagation of these species that can be replicated by small-scale growers and integrated into restoration processes.
MATERIAL AND METHODS
Study area. This research was conducted in the nursery of Botana experimental farm of Universidad de Nariño, located in the foothills of Galeras volcano, Pasto (Nariño) at 2820 masl. at 01°09’40.6”N and 77°16’44.6”W. The locality has an average temperature of 13°C, an average annual precipitation of 945mm, and 82% relative humidity (IDEAM, 2018). This area is classified as a lower montane moist forest (LM-f), according to the Holdridge´s life zones system (1990).
Species identification and selection. Species selection was based on cultural, ecological, and/or economic criteria of local growers. Likewise, the selection was also based on the study by Bacca and Burbano (2018) focused on the floristic composition of the High Andean ecosystem, which prioritizes the study of species suitable for ecologic restoration of the High Andean forest in Botana experimental farm.
The following species were selected for this study: O. floribundum, W. tomentosa, T. fuliginosa, and V. triphyllum, which belong to the families Araliaceae, Cunoniaceae, Boraginaceae, and Caprifoliaceae, respectively. Particularly, these families are used in the re-establishment of degraded areas, ecological restoration, and revegetation (Bacca and Burbano, 2018; Díaz and Velásquez, 2015; Hernández-Pineda et al., 2014; Toro, 2010). Furthermore, these species are acknowledged by local experts and have a cultural value.
Seed collection. The seeds used in this study were collected between October and November of 2016. The best individuals were identified during forest visits, resulting in the selection of 10 healthy, vigorous, and accessible trees per species. Mature and healthy fruits were collected from the ground or directly from the tree canopy using an extension pole. The fruits were stored in paper bags at room temperature and constant humidity.
To extract the seeds, fruit processing was performed according to fruit morphology (i.e., fleshy or dry) (Di Sacco et al., 2018; Espitia-Camacho et al. 2018;Torres et al. 2017). 1. Fleshy fruits: V. triphyllum and T. fuliginosa have purple and white round drupe fruits, respectively, at maturity stage. O. floribundum has purple round berry fruits. These fruits were manually opened under water; the seeds were washed and dried on paper at current air temperature. This process was performed immediately after fruit collection to avoid seed fermentation and damage. 2. Dry fruits: W. tomentosa has capsules that are easily opened by shaking (Di Sacco et al., 2018).
Seed viability was determined by the floatation technique which consisted of separating and discarding floating seeds. This technique also allowed extracting unwanted particles (Di Sacco et al., 2018; Oliva et al., 2015).
Evaluation of germination. The evaluation began in December 2016 until February 2017. The seeds were subjected to different hydration pre-germination managements associated with fundamental factors such as temperature and imbibition time, which were conducted in a recipient with 400mL of water under controlled conditions (Table 1) (Sánchez and Furrazola, 2018). The substrates varied regarding the ratio of sand and soil to obtain differences in filtration and humidity (Table 1). Before the application of the pre-germination management, the substrates were disinfected with boiling water. The seeds were planted in polyethylene bags with dimensions 3.5 x 6 x 1cm at a depth of three times the seed diameter and watered daily (Aguilar and Vanegas, 2009; Di Sacco et al., 2018; Lazos-Monterrosa et al., 2015).
Table 1. Substrates and pre-germination treatments used to evaluate the germination percentage of four native species.
| Substrate (S) | Pre-germination treatments (PM) |
|---|---|
| S1: soil (100%) | PM1: imbibition for 48h - T°22 °C |
| S2: sand (100%) | PM2: imbibition for 24h - T°22 °C |
| S3: soil + sand (50%-50%) | PM3: imbibition for 5min - T°80 °C |
| Control | PM4: without imbibition |
Experimental design. For each species, a completely randomized design was established with treatments distributed into split-plots. The main plot included the substrates (S) and the sub-plots included the different pre-germination treatments (PM) with three repetitions. The experimental sub-unit consisted of 20 seeds, for a total of 960 seeds per species and 3840 seeds total for the experiment (Di Sacco et al., 2018).
Germination percentage (%G) (equation 1) was assessed to determine the effect of the substrates and seed managements on germination capacity (Lazos-Monterrosa et al., 2015).e1
The observations consisted of weekly recordings until the number of germinated seeds was constant. The seeds germinated when the radicle emerged through the seed tegument and appeared on the substrate (Di Sacco et al., 2018; Lazos-Monterrosa et al., 2015; Sánchez et al., 2015).
Statistical analysis. Data was tested for normality using the Shapiro-Wilk test. Additionally, an analysis of variance (ANOVA) was performed, followed by Tukey’s multiple comparison test using Infostat® V.2019 statistical program. Significant values were established at P-values below 0.05 (P< 0.05) (Zar, 2010).
RESULTS AND DISCUSSION
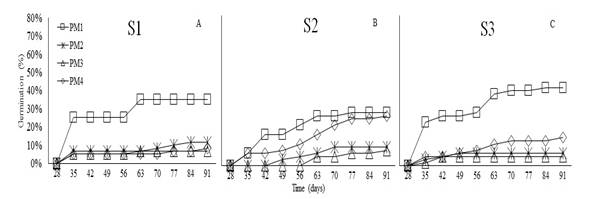
Germination. Germination of O. floribundum was measured at 35 days after sowing in the three substrates. This species showed germination percentages of 25 and 23% for PM1 in S1 and S3, respectively, and 7% for PM1 and PM4 in S2 (Figure 1). These findings confirm that morphological seed dormancy occurred in O. floribundum since a minimum of 28 days were required for onset of germination (Sánchez et al., 2019). At 91 days, the final germination percentages were 42% in S3, 35% in S1, and 28% in S2 with PM1.
The analysis of variance did not show statistical differences in the germination percentage (%G) of O. floribundum across substrates (P<0.4823); therefore, these factors do not affect germination. Conversely, significant differences were found in response to pre-germination managements (P<0.0193). Figure 2 shows that PM1 promoted the best germination percentages compared with the other pre-germination treatments. According to Sánchez et al. (2019), the family Araliaceae requires little sunlight and moist stratification for one or two months to reach 30% germination. Conversely, this study showed that up to 42% germination can be obtained by imbibition for 48 hours, while a temperature increase can reduce germination to 7%; thus, classifying these seeds as sensitive to heat stress. Therefore, if these seeds are left under direct sun exposure during planting, it can hinder their establishment; similar findings were established by Sánchez et al. (2015) in their study using autochthonous species of the same family.
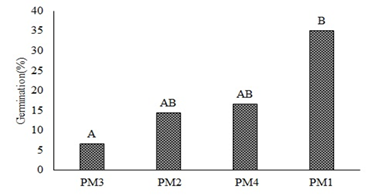
Figure 2. Germination percentage of O. floribundum in response to pre-germination treatments PM1, PM2, PM3, PM4. Tukey’s multiple comparison test (P< 0.05).
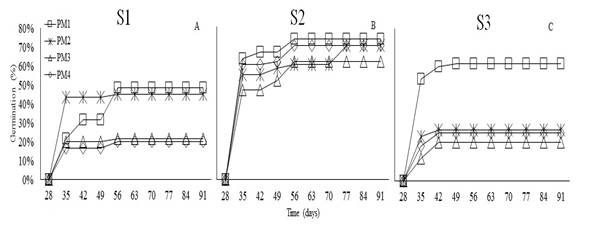
The germination in W. tomentosa began at 35 days after sowing in the three substrates (Figure 3). During this period, PM2 in S1 reached a germination percentage of 42%, PM1 in S2 achieved 60% germination, and PM1 in S3 obtained 53% germination. This study demonstrates that seed dormancy in W. tomentosa is broken by imbibition for 48h and sand substrate. Sánchez et al. (2019) mention that the family Cunoniaceae displays physiological dormancy with permeable seed coats and developed embryos (Baskin and Baskin, 2014; Sánchez et al., 2018b).
The analysis of variance did not show statistical differences in the germination percentage (%G) of W. tomentosa in response to pre-germination treatments (P<0.1207). However, significant differences in %G was observed across substrates (P<0.0038). Figure 4A shows that utmost germination percentage (68%) was achieved in S3, which showed a percentage of 74%. Regarding, S2 the highest percentage of germination was reached after 91 days, in all pre-germination treatments, ranging between 61 and 74% (Figure 4B). These values were higher than those reported by Romero (2015) for the same species, who observed germination percentages up to 45% in sand substrate. Moreover, Aguilar and Vanegas (2009) suggest an imbibition time of 24h on this same substrate. Siura and Moreno (2016) mention that sand substrate can promote germination since it is inert and provides better contact water-seeds; additionally, sand allows water to filter easily, which prevents phytosanitary issues.
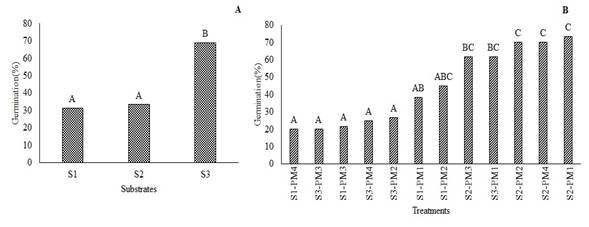
Figure 4. Germination percentage of W. tomentosa. A: substrates; S1, S2, S3. B: treatments (S-PM). Means with the same letter are not significantly different from each other (P<0.05).
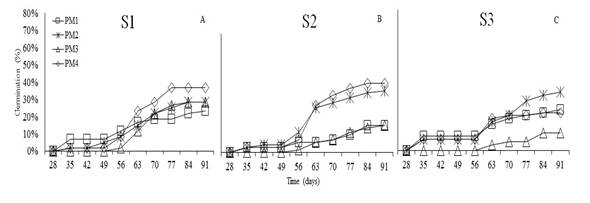
T. fuliginosa started to germinate at 35 days after sowing (Figure 5). The initial germination percentage was 7% with S1 and S3, and 3% with S2 using PM1. By the end of the experiment, PM4 reached the highest value (47%) in S2, followed by S1 with PM4 (37%) and S3 with PM2 (33%).
The analysis of variance showed no statistical differences in the germination percentage (%G) of T. fuliginosa in response to substrates (P<0.3261) and pre-germination treatments (P<0.1090). Sánchez et al. (2019) state that species of the family Boraginaceae are tolerant to temperatures between 25 and 35°C; furthermore, to achieve efficient germination, the seeds must be wrapped up in water for 12h and scarified to break physiological dormancy. However, according to this research, T. fuliginosa seeds germinate without pre-germination management (47%), and with imbibition for 24h on sand substrate, it reaches a germination of 42%. This can be explained since the species easily adapts to several environments, such as the edges of water bodies with sandy soils. (Sánchez and Furrazola, 2018; Toro, 2010). Moreover, Guerra et al. (2014) mention that sand substrate provides the necessary conditions for germination by avoiding excess of water and fungal growth.
Viburnum triphyllum seeds did not respond to the treatments (0%G). In this case, imbibition and warm stratification might have inhibited germination. Specifically, the hardness of the seed hull may have caused mechanical resistance to imbibition and embryo emergence. Therefore, different pre-germination treatments should be applied to break seed dormancy in this species. In most tree and shrub species, scarification is recommended to eliminate seed skin impermeability and overcome the physiological component that causes dormancy (Sánchez et al., 2019). Another pre-germination treatment can involve exposure of seeds to thermoperiods that resemble the effect of climate variability, which promotes germination in several species. Similarly, treatment with sulfuric acid (H2SO4) can be applied to simulate the action of the gastric acid of birds and mammals that disperse this type of seeds, thus, eliminating impermeability and breaking physiological dormancy (Acero-Nitola and Cortés -Pérez, 2014; Espitia et al., 2016; Sánchez and Furrazola, 2018; Sánchez et al., 2015; Sánchez et al., 2019). Nevertheless, the reproduction of many wild tree species is highly complex since dormancy is a natural survival mechanism and poses a challenge to manipulate germination (Sánchez et al., 2015).
Overall, this study contributes to the scientific knowledge on the germination of e four native species. The germination percentages were highly variable, ranging from 42 to 74% for O. floribundum and W. tomentosa, respectively. This variability is likely attributed to morphological and physiological responses of seeds to substrates and pre-germination treatments. Furthermore, the environmental conditions of site and the collection of seeds directly from the wild forest (i.e., without silvicultural management or genetic improvement) can also contribute to this variability (Tomášková et al., 2014). Mancipe-Murillo et al. (2018) reported different germination percentages, ranging from 25 to 100%, for native species from the High Andean Forest.
Palomeque et al. (2017) explained that low or null germination percentages could be associated with effective pollination, in which the mother tree displays floral asynchrony, or a high percentage of flowers that do not receive pollen, leading to low propagation. Some species have seeds with embryos that are morphologically developed but physiologically immature, which can affect germination capacity (Courtis, 2013); however, dormancy decreases when maturity is reached. Finally, many wild species inhabit extreme environments that affect seed production and cause low viability (Barrett-Lennard et al., 2016; Dayrell et al., 2016).
CONCLUSIONS
Germination of O. floribundum is affected by the pre-germination treatment. The highest germination percentage (42%) for this species was achieved with seed imbibition for 48h at 22°C.
Seed propagation of W. tomentosa achieved a germination percentage of 74%, with imbibition for 48h at 22°C and sand substrate. This finding constitutes a propagation alternative for this species.
The treatments and substates used here did not affect the germination of T. fuliginosa; however, germination percentage was 47%.
The substrates and pre-germination treatments applied to V. triphyllum did not stimulate seed germination. Therefore, additional research should consider scarification or thermoperiod treatments.