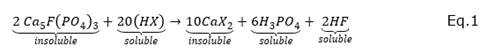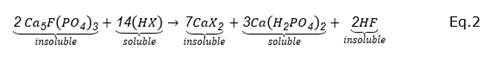INTRODUCTION
Agriculture consumes large amounts of phosphorus for soil fertilization using phosphate rock extracted from deposits. According to the 2017 reports from the USGS (United States Geological Surveys) (USGS, 2017) and the FAO (Zapata and Roy, 2007), it is estimated that in about 50 years, between 2040 and 2060, the conventional sources of phosphate rock (PR) will begin to be scarce. As well in about 100 years, the mines with easy access to this mineral would be exhausted (Gilbert, 2009). Thus, promoting the exploitation of less profitable mines (Restrepo et al., 2015). However, current fertilization practices that have a phosphorus application efficiency of less than 30% (Goldstein et al., 1993) could affect a possible crisis scenario in agricultural production (Cordell, 2010; Cordell et al., 2009; Walan et al. 2014; Edixhoven et al., 2013)
Microorganisms secrete various metabolites within their metabolic cycle as organic acids capable of solubilizing phosphorus from insoluble forms of phosphorus minerals as PR (Rashid et al., 2004). These acids (D-malic, D-lactic, L-malic, L-lactic, acetic, citric, oxalic, gluconic, Iyyappan et al., 2018; Li et al., 2015) act as ligands of sequestering cations bound to phosphate groups in a mechanism called chelation.
In fact, these acid metabolites play determining roles in the rhizosphere dynamics and jointly influence the solubilization of phosphates from soil minerals (Wakelin et al., 2004). The fungi Aspergillus niger and Penicillium Sp are potentially usable as phosphate solubilizing microorganisms (PSM) on an agro-industrial scale (Yin et al., 2015; Mendes et al., 2015) because these fungi are practically ubiquitous species in the natural environment , and they are characteristic of Colombian soils (Pérez et al., 2012). However, there is a great variety of species around the world.
In addition, these fungi have the ability to withstand environmental conditions and difficult substrates for other species, which implies that they have adaptive mechanisms to feed on unconventional sources of nutrients, and they generate specialized substances capable of carrying out these metabolic processes (Kaur and Reddy, 2017). For example, studies of deficient phosphorus soils (as Amazon basin soils and moor’s acid soils) several phosphorus-solubilizing microorganisms, that have allowed the conservation of biota have been isolated (Vera et al., 2002; Lizarazo and Vásquez, 2015).
A well-studied reaction scheme in the solubilization of phosphate, using microorganisms via the formation of metal complexes of Ca+ 2, is presented in equations 1 and 2 (Ivanova et al., 2006). In these reactions, Ca+ 2 is presented as a main component of apatites (or phosphoric rocks), and organic acids such as oxalic acid and citric acid that form calcium oxalate and calcium citrate, respectively. These acids are common metabolites produced by species of the fungi Penicillium sp and Aspergillus niger (Visagie et al., 2014) and are recognized as PSM (Posso and De Prager, 2017).
Where XH can be citric ( C 6 H 8 O 7 ) or oxalic acid ( 𝐶 2 𝐻 2 𝑂 4 )
Equations 1 and 2 show the organic acid’s action in forming poorly soluble compounds namely as calcium chelates, CaX2, and soluble compounds; calcium diphosphate as an intermediate product (superphosphate) and phosphoric acid (H3PO4). In these processes, the phosphate ion is released from the solid substrate by the chelating action of citric or oxalic acids on the Ca+2 cation (Mai et al., 2016). The power of complex-forming acids (chelates) consists in the action of oxygens (more electronegative) of the carboxyl groups that surround the calcium cation (Harvey, 2000). In this way, they can solubilize insoluble compounds as found in apatite.
The global trend is oriented towards scientific advancement for the development of innovative technologies that make agribusiness sustainable (Zapata and Roy, 2007), and the use of microorganisms to improve the solubility of phosphates in phosphate rock has been suggested in different works (Restrepo et al., 2015; Stutter et al., 2012; Li et al., 2015). For this reason, there are different advances in soil bioremediation (Pineda, 2015) and in biofertilization (Pérez et al., 2012).
Therefore, the Aspergillus niger and Penicillium sp species were used in the formulation, application and evaluation of treatments that allow solubilizing phosphates from phosphate rock samples taken from the Media Luna mine in Aipe- Huila (Colombia), one of the places where phosphate rock is extracted.
MATERIALS AND METHODS
Phosphoric Rock. The ground phosphoric rock samples were collected from the Media Luna mine in Aipe, Huila, with coordinates: Latitude 3°54'0.50184"N, Longitude 75°19'32.0592", 20km from the municipal seat. The samples were sieved in the laboratory and the fractions with particle diameters between 125 - 500µm were worked on.
Coffee pulp substrate (stillage). The biological treatment was carried out in an aqueous medium, with aeration, and coffee pulp (Coffea arabiga) was used as substrate; material selected for its content of carbohydrates and essential nutrients (Silva et al., 2013; Serrat et al., 2018) due to the recognized problem as a residue in coffee production (Beyene et al, 2012). Fresh coffee pulp from the rural area of San Agustín was obtained with coordinates: Latitude 1°54'05.9760” N, Longitude: 75°17'06.9828”W.
The coffee pulp was collected from the coffee pulping process, part of the coffee mill in which the pulp is generally discarded as waste. Later, the coffee pulp was stored under refrigeration and transferred to laboratory. In laboratory, the pulps were macerated with distilled water, filtered, sterilized in an autoclave (model NO75X) for 20min at 120°C and 1.0MPa.
No additional nutrient was added. This preparation will be referred to as vinasse due to its richness in fermentable sugars and its easy biodegradation by microorganisms. The pH measured in the stillage was 4.0, which was not modified to sow of the inocula.
Colonies of Aspergillus niger and Penicillium sp. 20mL of PDA (Potato Dextrose Agar) were poured into 90mm Petri dishes. The boxes were then exposed to the environment for 30 minutes to collect the microorganisms presented. The mushrooms’s capture was made in the urban area of Neiva-Huila with coordinates (2°55'50.85''N, 75°15'54.503''W), with a warm tropical climate and average daytime temperatures of 28°C.
Then, the Petri dishes were sealed and incubated in the dark for 7 days at an average temperature of 29°C. After this time, the Petri dishes were selected in which, possible colonies of Aspergillus niger and Penicillium sp were identified according to the Visagie (2014) methodology by observing their macroscopic characteristics. Then, under aseptic conditions with a controlled environment in a laminar flow cabinet, colonies of individual species were sown from the cultures previously obtained and identified in Malt Extract Agar (MEA). Finally, the macroscopic and microscopic identification of the species was made using an Olympus model microscope by comparing their morphological characteristics, observing the outcrop of conidiophores, and the formation of hyphae inoculated fungi.
Evaluation of the treatments with stillage, Aspergillus niger, and Penicillium sp. The applied treatments V, VA, VP, VAP, and H40 are described in Table 1 for a 2x2 factorial design, for a total of 15 experimental units where the composition of the treatment was varied in a controlled temperature of 30.6°C. The variable evaluated was the composition of the mixture, conserving the proportions of RF and stillage used, but changing the mass ratio of the fungi, and the observable was the dissolved phosphate for several days of incubation.
Table 1. Description of the biological treatments used for the solubilization of phosphorus.
| No. | Treatment (B) | Descripción | Mass ratio of Aspergillus niger: Penicillium sp. |
|---|---|---|---|
| 1 | V | PR + stillage | (0:0) |
| 2 | VA | PR + stillage + Aspergillus niger | (1:0) |
| 3 | VP | PR + stillage + Penicillium sp. | (0:1) |
| 4 | VAP | PR + stillage+ Penicillium sp. + Aspergillus niger | (1:1) |
| 5 | H40* | PR+ 𝐻 2 𝑆 𝑂 4 40%𝑣/𝑣 (Reference) | ------ |
* Biological treatment
The change in pH was controlled with the addition of CaCO3 and all experiments were done by triplicate. In the treatments, the coffee pulp (Coffea arabiga) was used as a substrate due to its easy obtaining as a by-product in the Coffee production (Puerta and Ríos, 2011; Serrat et al., 2018). The treatments were carried out in a controlled environment (Figure 1) with aeration during intervals of two hours per day.
Figure 1. Configuration of the treatment aeration system a) Scheme b) Photograph of an aeration system.
Dissolved phosphate was determined by colorimetry. 7mL samples were taken. They were centrifuged for 30min at 5000rpm to eliminate the high turbidity and the solids particles. Then, 5mL aliquots were taken and diluted to 50mL with distilled water. These solutions were treated with aluminum sulfate crystals to remove the color caused by the biological remnant and were centrifuged for 15 min to remove the flocs formed.
Finally, 10mL of the clarified solution was mixed with 2.5mL of vanadate-molybdate reagent (RVM), and the absorbance at 400nm wavelength was measured in a SPECTROQUANT model PHARO 300 spectrophotometer.
The results were correlated with the curve of monopotassium phosphate standards, previously prepared and valid for a range of 0 to 50 ppm of phosphate in the aqueous phase.
Data analysis. The Bartlett test was performed to establish the normality of the data (p> 0.05) checking the assumptions of normality and homoscedasticity, and performing an analysis of variance by ranges for non-parametric distributions with the Kruskal-Wallis test. The null hypothesis was evaluated (for each case of interest) with a significance level of 5%. Matlab R2016a, Inc. software was used for statistical analysis (Mathworks, 2019a; Mathworks, 2019b; Mathworks, 2019c; Mathworks, 2019d).
RESULTS AND DISCUSSION
Table 2 shows the percentage of total dissolved phosphorus achieved with the treatments and H2SO4 (40% v/v). The V, VA, VP, and VAP treatments were positive in the phosphate dissolution of the phosphate rock, the VAP treatment being the most prominent, 7.8% of the phosphorus solution obtained with H2SO4 (40% v/v). All treatments showed significant differences among themselves for 42 days of incubation.
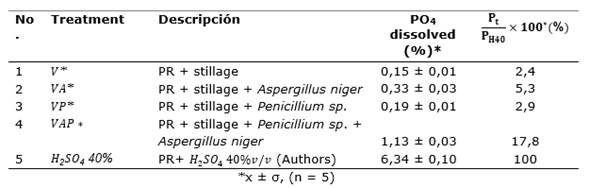
Table 2. Dissolved PO4 values obtained for each biological treatment for 42 days of incubation at 30°C.
Whereas the dissolved phosphate concentrations (Table 3) for the VA and VAP treatments increased significantly as the incubation days passed, the phosphorus concentrations with the V and VP treatments decreased from the first week of incubation. The highest phosphate concentration was for the VAP treatment (140 ± 17.1mg/L) and the lowest concentration was with the V treatment (13 ± 1.5mg/L).
Table 3. Average concentrations of soluble P (mg/mL) for each of the biological treatments according to the days of incubation.
| Treatments | Concentration of P (mg/mL)/Days of incubation* | |||||
|---|---|---|---|---|---|---|
| 0 | 5 | 12 | 18 | 25 | 31 | |
| V | 28 ± 0,9 | 19 ± 12,9 | 16 ± 2,6 | 13 ± 1,5 | 18 ± 3,0 | 19 ± 4,8 |
| VA | 23 ± 1,8 | 29 ± 3,4 | 47 ± 7,0 | 41 ± 4,7 | 34 ± 1,0 | 35 ± 2,5 |
| VP | 26 ± 2,0 | 22 ± 6,1 | 25 ± 2,7 | 21 ± 3,3 | 19 ± 2,6 | 18 ± 1,6 |
| VAP | 31 ± 5,1 | 103 ± 31,6 | 127 ± 21,2 | 140 ± 17,1 | 134 ± 25,0 | 127 ± 17,2 |
*n = 5, x ± σ
The phosphate concentrations are similar to other values (201.3-283.7mg/L) reported in similar works (Chunquiao et al., 2015) with solubilizing fungi e.g Aspergillus niger and Aspergillus carbonarius. The authors incorporated ammonium and nitrate to the culture medium as additional nutrients or to the soluble phosphate concentrations (284.7 - 366.6mg/L) reported in a work carried out with the genetically modified Aspergillus niger fungus (Cássia et al., 2014).
Significant differences were found between the data groups of each treatment for each week of study with a significance level of 0.05, applying the Kruskal-Wallis test (Table 4).
Table 4. Kruskal -Wallis hypothesis test for a significance level of 0.05 for the treatments during different days of incubation.
| Caso | Assumed hypothesis | H<X 2 |
|---|---|---|
| i. | Ha: There is a significant difference in PO4 -3 concentration between different treatments for day 1. | 0,119<5,87 |
| ii. | Ha: There is a significant difference in PO4 -3 concentration between different treatments by day 5. | 0,0415<8,23 |
| iii. | Ho: There is a significant difference in PO4 -3 concentration between different treatments by day 12. | 0,01530<10,42 |
| iv. | Ho: There is a significant difference in PO4 -3 concentration between different treatments by day 18. | 0,01560<10,38 |
| v. | Ha: There is a significant difference in PO4 -3 concentration between different treatments by day 25. | 0,0227<9,56 |
| vi. | Ha: There is a significant difference in PO4 -3 concentration between different treatments by day 31. | 0,0230<9,53 |
The increase of the micellar diameter was a result of the fungi’s growth. Aeration allowed constant mixing which contributed to abundant hyphal growth and sporulation in the second week. In all treatments, the formation of supernatant gelatinous masses, or also submerged in the substrate, was observed mainly composed of mycelia of fungi and organic matter. In some treatments, changes are observed in the color of the liquid phase, which turned dark. In addition, samples of the treatments were periodically observed under the microscope, and the presence of inoculated fungi was verified.
In the VP treatment, there was little growth of Penicillium sp colonies, in the form of thin superficial films (approx. 1mm) that showed slight sporulation. In VA, there was a growth of Aspergillus niger colonies, in the form of superficial films (1-2mm), and abundant sporulation with a darker coloration. In VAP, the majority growth of Aspergillus niger colonies (over those of Penicillium sp), was observed covering almost the entire surface in the form of superficial films of great thickness (10-30mm) with abundant sporulation and with small islands of colonies of Penicillium sp; also, sporulated (less than 10mm in diameter). On a microscopic scale, the growth of the characteristic conidiophores inoculated in VA, VP, and VAP was periodically observed, as well as the gelatinous films formed in which an abundant growth of hyphae was identified.
The metabolism the Aspergillus niger and Pencillium sp. fungus excretes organic acids. The increase in pH can be explained by different causes including the dissolution of carbonates and / or hydroxyl present in RF. There is also the probability that there was ammonia production because of the unwanted presence of bacteria (Beyene et al., 2012; Peterson et al., 2005).
According to the results, it can be inferred that Aspergillus niger presented a better adaptation to the substrate in the process conditions than Penicilium sp. Interestingly. The highest dissolution values were reached in the combined VAP treatment, which may a possible synergistic activity between the two species. Nevertheless, the predominant advance of Aspergillus niger was evidenced macroscopically. An interesting feature in the tests where Aspergillus niger thrived was the black staining of the aqueous phase, possibly due to the high production of spores; this characteristic coincided with a higher dissolution of phosphates. Finally, it was found that these are a suitable medium for the growth of the fungi Aspergillus niger and Penicillium sp concerning stillage; following what is suggested by other authors (Ivanova et al, 2006), even at low pH values.
CONCLUSIONS
All the biological treatments applied in this study contributed to the solubilization of phosphates in phosphate rock with significant differences between them. The treatment that included the mixture of fungi Aspergillus niger - Penicillium sp reached the highest concentration of soluble phosphate (1.13 ± 0.03), achieving 17.8% of the solution obtained with concentrated sulfuric acid ( 𝐻 2 𝑆 𝑂 4 - 40%).