INTRODUCTION
Since long before, insects damage grains under storage both qualitatively and quantitatively. To reduce these losses, several synthetic chemicals have been developed and used. But continuous and uncontrolled uses of these chemicals have caused several health and environmental problems. Unintentional use of insecticides is killing over 355,000 people per each year (Alavanja and Bonner, 2012). Besides, agrochemical residues contaminate land ecosystems and cause food poisoning (EEA, 2013). Among them, organochlorines are persistent in soils and sediments and accumulate in nonhuman organisms with adverse outcomes at population level (Köhler and Triebskorn, 2013). These residues cause mass killings of bees, birds, amphibians, fish and small mammals (W.H.O, 2017). Thus, public issues have diverted towards the use of plant volatiles in insect pest management.
Aromatic essential oil is produced as secondary metabolites by the plants of Alliaceae, Apiaceae, Asteraceae, Cupressaceae, Lamiaceae, Lauraceae, Myrtaceae, Piperaceae, Poaceae, Rutaceae and Zingiberaceae family. These essential oils are complex mixtures of different compounds in different concentrations. Each essential oil is characterized by specific fragrance due to its two or three major components. Composition of these oils depends on parts of plant used for extraction, extraction method, plant phenological stage, harvesting season, plant age, genotype of plant, soil nature and environmental conditions (Atti-Santos et al., 2004; Angioni et al., 2006; Verma et al., 2011). These oils have larvicidal, adulticidal, antifeedant, oviposition inhibitory activities, capacity to delay development and adult emergence (Caballero-Gallardo et al., 2011; Isman et al., 2011; Liu et al., 2011; Stefanazzi et al., 2011).
Anethum graveolens (Family: Umbelliferae) commonly known as dill has Mediterranean and Asian origin. Dill seeds have strong spicy odor and used as flavoring agent in salads, sauces, soups, tea, sea foods and pickles (Babri et al., 2012; Isbilir and Sagiroglu, 2011). Dill seeds are used as medicine in bladder inflammation, liver diseases and insomnia (Naseri and Heidari, 2007). Its volatile oil is used as antispasmodic agent, anticonvulsant, anti-emetic and anticramp remedy; and recommended topically as wound healer (Naseri and Heidari, 2007). It is a hypolipidemic and cardio-protective agent used in lowering blood cholesterol level (Hajhashemi and Abbasi, 2008). Dill seed essential oil contains carvone, dillapiole and limonene as major compounds (Hussein et al., 2015).
Illicium verum (Family: Magnoliaceae) commonly known as star anise is grown nearly 80% in the world (Cai et al., 2013). Its fruits are used as spice and in treatment of flatulence, spasmodic and colic pain. Its oil is used in the treatment of rheumatism and otalgia (Singh et al., 2007). This oil is effective against bacterial, yeast and fungal strains (Singh et al., 2007). Essential oil of star anise fruit has trans-anethole, estragole and limonene as major components (Padmashree et al., 2007). Limonene, an aliphatic and cyclic monoterpene is a colorless liquid and is the major constituent of essential oil of citrus fruits, such as lemons, limes, and oranges rind (Perez-Cacho and Rouseff, 2008). Limonene is also one of the major components of Anethum graveolens and Illicium verum essential oil (Padmashree et al., 2007; Hussein et al., 2015).
Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae) is a serious pest of maize and other cereals under storage (Demissie et al., 2008). It is common in humid tropical areas of the world where maize is cultivated. This insect attacks stored cereal products like wheat, rice, sorghum, oats, barley, rye, buckwheat, peas and cottonseed. This also infests other processed cereal products such as pasta, cassava and various coarse, milled grains. Weevil adults attack whole grains and larvae cryptically feed and develop within grains (Ileleji et al., 2007). In this study, essential oils isolated from A. graveolens seeds and I. verum fruits oils and one of its major constituents, limonene have been evaluated for their repellent, insecticidal, anti-oviposition and acetylcholinesterase enzyme inhibitory activities against maize weevil, S. zeamais.
MATERIALS AND METHODS
Insect rearing. Maize weevil, S. zeamais was used to investigate insecticidal properties of A. graveolens and I. verum oils. The insects were collected from the local market of Gorakhpur (U.P.) in the month of May and reared on whole maize grain at 28±40C, 50±5% RH and photoperiod of 10:14 (L:D) hours.
Essential oils. A. graveolens seeds and I. verum fruits were purchased from local market of Gorakhpur (U.P.). These were grounded and hydrodistilled in Clevenger apparatus (Standard Scientific Glass Industries, Mumbai, Maharastra, India) for six hours to isolate essential oil. After isolation, essential oils were kept in Eppendorff tubes (Torsons Products Pvt. Ltd, Kolkata, West Bengal, India) at 40C till further use (Chaubey, 2007). Pure Limonene was purchased from Sigma Chemicals (USA).
Contact toxicity. Formulations of A. graveolens and I. verum oils and pure limonene were made in acetone, applied on bottom surface of glass petri dish (diameter 8.5cm, height 1.2cm) and left for few minutes for evaporation of acetone. Ten adults (1-2 days old) taken from laboratory culture were released at the center of petri dish, covered and kept at 28±40C, 50±5% RH in dark. After 24 and 48 h of exposure, adult mortality was recorded (Chaubey, 2013).
Fumigant toxicity. Formulations of A. graveolens and I. verum oils and pure limonene were made in acetone. Ten adults (1-2 days old) taken from the laboratory culture were placed with 2g of maize grains in glass petri dish. Filter paper strip (2cm diameter) was treated with oil formulations and left for few minutes to evaporate acetone. Now oil coated filter paper was pasted on undercover of petri dish, air tightened with parafilm and kept 28±4°C, 50±5% RH in dark. Six replicates were set for each concentration of oil and control. After 24 and 48h of fumigation, adult mortality was recorded.
Repellent activity. Repellency assay was performed in glass petri dishes (diameter 8.5cm, height 1.2cm). Test solutions of A. graveolens and I. verum oils and pure limonene were prepared in acetone (Analytical Grade, Himedia, Mumbai, Maharastra, India). Whatman filter papers (Qualigen’s, Germany) were cut into two halves and each test solution was applied to filter paper half as uniform as possible using micropipette (Fine Care Corporation, Dantali, Gujarat, India). The other half of filter paper was treated with acetone only. Oil treated and acetone treated halves were dried to evaporate acetone completely. Both treated and untreated halves were then attached with cellophane tape in and placed in each petri dish. Forty S. zeamais adults (1-2 days old) were released at the center of filter paper disc and petri dish was covered and kept in dark. Six replicates were set for each concentration of oil. After 4 h of exposure, adults in treated and untreated halves were counted. Percent repellency (PR) was calculated using formula: PR = (C−T)/(C+T) ×100, C = number of insects in the untreated halves, and T = number of insect in treated halves. Preference index (PI) was calculated using formula:
PI between - 1.0 and - 0.1 indicate repellant oil, - 0.1 to + 0.1 neutral oil and + 0.1 to + 1.0 attractant oil.
Oviposition inhibition. Ten S. zeamais adults (1-2 days old) of mixed sex were fumigated with two sub-lethal concentrations viz. 40% and 80% of 24h LC50 and 48h LC50 of A. graveolens and I. verum oils and pure limonene for 24h and 48h respectively and reared on maize grain in 250ml plastic box for 10 days. After 10 days, adults were discarded and number of F1 progeny was counted after 45 days. Six replicates were set for each concentration of oil and control.
Acetylcholinesterase enzyme (AChE) activity determination. S. zeamais adults (1-2 days old) were fumigated with two sub-lethal concentrations viz. 40 and 80% of 24-h LC50 of A. graveolens and I. verum oils and pure limonene. After 24h of fumigation, adults were used for determination of enzyme activity (Ellman et al., 1961). Fumigated insects were homogenized in phosphate buffer saline (50 mM, pH 8) and centrifuged. Supernatant was used as the acetylcholinesterase source. To 0.1mL of enzyme source, added 0.1 mL substrate acetylthiocholine iodide (ATChI) (0.5 mM), 0.05 mL chromogenic reagent 5,5-Dithio-bis 2-nitrobenzoic acid (DTNB) (0.33mM) and 1.45mL phosphate buffer (50mM, pH 8). Acetylcholinesterase enzyme activity was determined by measuring changes in the optical density at 412nm by incubating the reaction mixture for 3min at 25°C. Enzyme activity was expressed as mmol of ʻSHʼ hydrolysed min-1 mg-1 protein.
Statistical analysis. One-way Analysis of variance (ANOVA, P<0.01), and correlation and linear regression analysis were conducted to define concentration-response relationship (Sokal and Rohlf, 1973). Median lethal concentration (LC50) was calculated using POLO programme (Russel et al., 1977).
RESULTS AND DISCUSSION
Contact toxicity. A. graveolens and I. verum essential oils and pure limonene caused contact toxicity in S. zeamais adults. Median lethal concentrations (LC50) were 0.219 and 0.159µlcm-2; and 0.269 and 0.226µlcm-2 area for A. graveolens and I. verum essential oils after 24 and 48h of exposure respectively (Table 1). For pure limonene, LC50 values were 0.567 and 0.386µlcm-2 after 24 and 48h of exposure respectively.
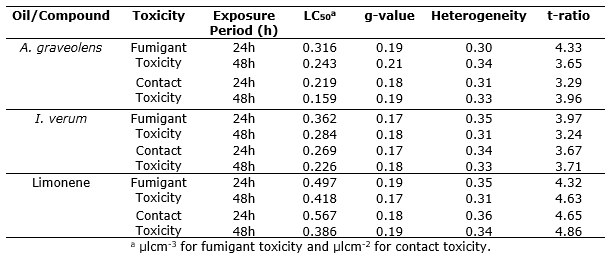
Table 1. Fumigant and contact toxicity of A. graveolens and I. verum oils and limonene against S. zeamais adults.
In the present study, repellent, toxic and oviposition inhibitory activities of A. graveolens and I. verum oils and pure compound limonene were studied against S. zeamais. Both essential oils repelled S. zeamais adults even at very low concentration. A. graveolens and I. verum oils and pure compound limonene killed S. zeamais adults by both contact and fumigant toxicity. Progeny production was reduced by inhibiting oviposition in S. zeamais when treated with A. graveolens and I. verum oils and pure limonene. Similar results were obtained when S. zeamais adults were exposed to sub-lethal concentrations of C. cyminum and P. nigrum oils (Chaubey, 2017a). Peumus boldus leaf oil has shown repellent and lethal actions against S. zeamais adults. This oil reduced F1 emergence in S. zeamais adults when fumigated (Betancur et al., 2010).
Fumigant toxicity. Fumigation of A. graveolens and I. verum essential oils and pure limonene killed S. zeamais adults by vapour action. Median lethal concentrations (LC50) were found 0.316 and 0.243 μlcm-3 air for A. graveolens oil after 24 and 48h of exposure respectively (Table 1). Similarly, LC50 values were 0.362 and 0.284 μlcm-3 air for I. verum oil after 24 and 48 h of exposure respectively. For pure limonene, LC50 values were 0.497 and 0.418 μlcm-3 after 24 and 48h exposure respectively. Regression analysis showed concentration-dependent mortality in S. zeamais adults by A. graveolens and I. verum oils (For A. graveolens oil, F = 259.74 for 24h and 269.89 for 48h; I. verum oil, F = 286.83 for 24h and 303.66 for 48h; and for pure limonene, F = 236.21 for 24h and 245.87 for 48h; P<0.01) (Table 2). The index of significance of potency estimation, g-value indicates that the mean value is within the limits of all probabilities (P<0.1, 0.5 and 0.01) as it is less than 0.5. Values of t-ratio greater than 1.6 indicated that the regression was significant. Values of heterogeneity factor less than 1.0 denotes that model fits the data adequate.
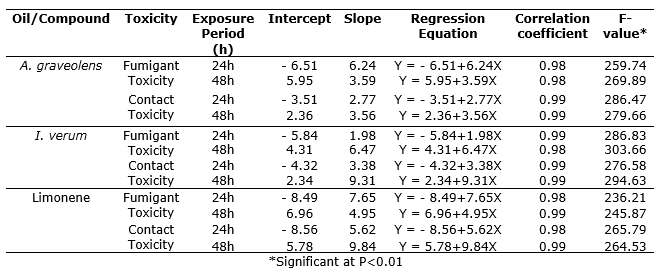
Table 2. Regression analysis of fumigant and contact toxicity of A. graveolens and I. verum oils and limonene against S. zeamais adults.
Acetylcholinesterase enzyme is found at postsynaptic neuromuscular junctions in muscles and nerves. It hydrolyzes a naturally occurring neurotransmitter acetylcholine into acetate and choline (McHardy et al., 2017). Thus, inhibition of acetylcholinesterase prevents inactivation of postsynaptic neurons thereby increasing both the level and duration of action of acetylcholine in the nervous system. Both A. graveolens and I. verum oils and limonene reduced acetylcholinesterase activity in S. zeamais when treated with sublethal concentration of oils/compounds. Similarly, fumigation of S. zeamais adults with C. cyminum and P. nigrum oils inhibited acetylcholinesterase activity (Chaubey, 2017a). Probably, normal mating and normal neurotransmission might be inhibited in S. zeamais adults by A. graveolens and I. verum oils and limonene. All these effects may be helpful in reducing damage by S. zeamais. Earlier studies have established insecticidal properties of plant volatile oils (Isman et al., 2011; Liu et al., 2011; Stefanazzi et al., 2011; Chaubey, 2012a; Chaubey, 2012b; Chaubey, 2012c; Chaubey, 2013; Chaubey, 2014; Chaubey, 2016a, Chaubey, 2016b). Piper nigrum, Cuminum cyminum, Allium sativum and Aegle marmelos essential oils show contact toxicity, fumigant toxicity, repellent, oviposition inhibitory and developmental inhibitory activities against S. zeamais (Chaubey, 2017a, Chaubey, 2017b). Fumigation of these essential oils has also been known to inhibit acetylcholinesterase activity in S. zeamais (Chaubey 2017a; Chaubey, 2017b).
Repellent activity. Maximum repellency and Preference Index (PI) were observed at 0.8% concentrations of A. graveolens and I. verum oils (Table 3). A. graveolens and I. verum oils and pure limonene showed significant (F = 253.93 for A. graveolens; F = 246.67 for I. verum; F = 238.64, P<0.01) repellency against S. zeamais adults.
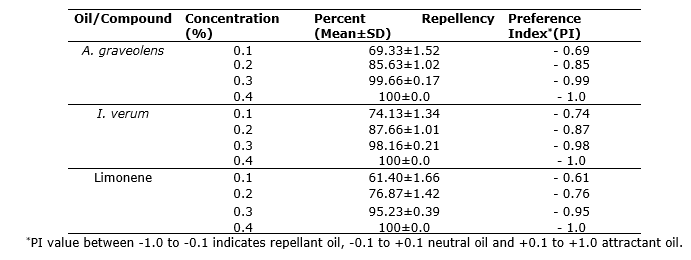
Table 3. Repellent activity of A. graveolens and I. verum oils and limonene against S. zeamais adults.
Oviposition inhibition. Fumigation of S. zeamais adults with A. graveolens and I. verum essential oils reduced oviposition potential. When S. zeamais adults were fumigated with 80% of 24h LC50 of A. graveolens and I. verum oils oviposition was reduced to 41.82% and 51.27% of control respectively (F = 218.89 for I. verum, and F = 254.94 for A. graveolens oils; P<0.01) (Table 4). Similarly, reduction in oviposition was 22.72% and 31.34% of control when S. zeamais adults were fumigated with 80% of 48h LC50 of A. graveolens and I. verum oils respectively (F = 255.84 for A. graveolens oils and F = 287.85 for I. verum; P<0.01). In case of pure limonene, reduction in oviposition was 80.31% and 55.49%; and 68.54% and 41.16% of control when S. zeamais adults were fumigated with 40% and 80% of 24h and 48h LC50 respectively (F = 237.21 for 24h and F = 245.39 for 48h; P<0.01).
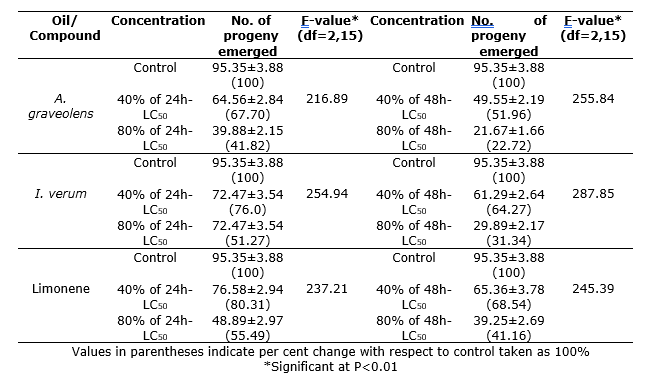
Table 4. Oviposition inhibitory activities of A. graveolens and I. verum oils and limonene in S. zeamais.
Acetylcholinesterase enzyme activity. Both A. graveolens and I. verum oils inhibited acetylcholinesterase activity in S. zeamais adults significantly. Fumigation of S. zeamais adults with 80% of 24h LC50 of A. graveolens essential oil reduced acetylcholinesterase activity to 54.47% of control respectively (F = 198.37; P<0.01) (Table 5). Similar treatment of S. zeamais adults with I. verum essential oil significantly reduced acetylcholinesterase activity to 50.40% of control (F = 216.09; P<0.01) (Table 5). When S. zeamais adults were fumigated with 80% of 24h LC50 of limonene, acetylcholinesterase activity was reduced to 48.37% of control (F = 195.68; P<0.01).
Table 5. Effect of A. graveolens and I. verum oils and limonene on acetylcholinesterase enzyme activity in S. zeamais.
| Oil/Compound | Concentration | Enzyme activity* | F-value (df=2,15)# |
|---|---|---|---|
| A. graveolens | Control | 0.0984±0.0029(100) | 198.37 |
| 40% of 24h-LC50 | 0.0756±0.0002(76.82) | ||
| 80% of 24h-LC50 | 0.0536±0.0016(54.47) | ||
| I. verum | Control | 0.0984±0.0029(100) | 216.09 |
| 40% of 24h-LC50 | 0.0697±0.0018(70.83) | ||
| 80% of 24h-LC50 | 0.0496±0.0014(50.40) | ||
| Limonene | Control | 0.0984±0.0029(100) | 195.68 |
| 40% of 24h-LC50 | 0.0709±0.0002(72.05) | ||
| 80% of 24h-LC50 | 0.0476±0.0018(48.37) |
*mmol of ʻSHʼ hydrolysed min-1 mg-1 protein. Values in parentheses indicate per cent change with respect to control taken as 100%
#Significant at P<0.01
Besides volatile oils, its individual components have also been known for its effectiveness against insects (Chaubey, 2012a, Chaubey, 2012c; Ogendo et al., 2008). Pure compounds like linalool and linalyl acetate have been shown to cause toxicity in rice weevils (Ogendo et al., 2008). Menthol, methonene, limonene, α-pipene, β-pipene, β-caryophyllene and linalool show acetylcholinesterase inhibitory activities (Enan, 2005; Chaubey 2012a). The rapid action of volatile oils and constituents in insects indicates neurotoxic mode of action. Most insecticides bind to receptor proteins and interrupt normal neurotransmission leading to paralysis and death in insects. Recent studies suggest that low molecular weight terpenoids with different structures may also bind to target sites on receptors that modulate nervous activity (Hollingworth et al., 1984). Low molecular weight terpenoids are lipophilic in nature and cross the cuticle to reach the haemolymph and the proposed route of entry is tracheae (Veal, 1996).
These oils and pure compounds interference with neuromodulator octopamine (Enan, 2005) or GABA-gated chloride channels (Tong and Coats, 2012). Several oil and its components have been found to act on the octopaminergic system of insects. Octopamine is a neurotransmitter, neurohormone and circulating neurohormone-neuromodulator. Disruption in its activity breaks down of the nervous system in insects (Hollingworth et al., 1984). Most essential oils and its components bind to acetylcholinesterase enzyme and interrupt normal neurotransmission leading to paralysis and death (Chaubey, 2017a; Chaubey, 2017b). The mode of action of monoterpenes is still not known.
CONCLUSION
From the present investigation, it can be concluded that A. graveolens and I. verum oils and one of its major constituent limonene show excellent repellency and cause death and reduction in progeny production by inhibiting oviposition. These oils and compound also inhibit activity of acetylcholinesterase in insects. All these effects may be helpful in reducing damage by S. zeamais. Furthermore, the outcomes of the study will help in formulating plant based insect pest controlling preparations for stored grain insect pest management.














