INTRODUCTION
The soil has a large number and diversity of microorganisms responsible for its biological activity. The quality and quantity of plant material it has and the climatic factors affect microbial abundance, biodiversity, and their trophic relationships (Wright and Coleman, 2000). The microbial activity of the soil constitutes a measure of ecological importance, since on the one hand it represents the level of biological activity that involves the labile component of organic matter and, on the other, it integrates environmental factors and their influence on it (Zagal et al., 2002; Wing Ching-Jones and Uribe Lorío, 2016).
Nutrient recycling is influenced by the quality and quantity of plant material that enters the soil and by climatic and edaphic characteristics which also affect microbial abundance, the involved species, and their trophic composition (Wright & Coleman, 2000; Di Ciocco et al., 2014). This means that the biological activities are bioindicators of soil quality (Alvear et al., 2007; Jaurixje et al., 2013).
Soil is a non-renewable natural resource, exposed to biotic and abiotic factors. The biological activity of soils is regulated by numerous enzymes and the microbial habitat and plant roots are affected by them (Makoi and Ndakidemi, 2008). The enzymes come from living organisms. Once they die, some resist and retain their activity in the soil for long periods. Soil enzymes not only play an important role in the chemical and biochemical environment, but they also have an effect on how fast nutrients are available to plants (Kannaiyan and Kumar, 2009).
All biological process occurring in the soil are enzymatic reactions (mineralization, immobilization, nitrogen fixation, nitrification, etc.); that is why the Total Biological Activity (TBA) is considered to be an index of soil fertility (Burns, 1982; Alvear et al., 2007).
There are numerous physical, chemical, biological, or biochemical factors to assess soil quality; these act as quality indicators. One of the biochemical parameters available to evaluate the biological activity of the soil is known as hydrolysis of fluorescein diacetate (FDA) (Adam and Duncan, 2001; Alvear et al., 2007; Perez et al., 2015). This measure of the enzymatic activity can be used to infer the microbiological activity of certain soils (Lillo et al., 2011), and it is a sensitive parameter for resource management practices (Makoi and Ndakidemi, 2008).
FDA is broken down by numerous enzymes, such as proteases, lipases, and esterases. Therefore, its hydrolysis is considered to be a general or broad spectrum indicator of biological soil activity (Bandick and Dick, 1999). The product of the enzymatic reaction is fluorescein, which can be observed under a fluorescence microscope, or it can be measured using a spectrophotometer (Green et al., 2006). For some time now, FDA hydrolysis has been used as an indicator of the active global microbial activity of soils under different types of management in order to determine which system favors global microbial activity (Alvear et al., 2007).
The cultivation of yacón (S. sonchifolius) dates back to pre-Columbian times. It has adapted to the ecological conditions of the Andean region, and it has been linked to the traditions of the people living there (Fernández et al., 2006; Mansilla et al., 2010). In recent years, it has gained interest for its nutraceutical properties, since it is a natural prebiotic, and also for having tubers rich in inulin (Niziol-Lukaszewska et al., 2010; Arnao et al., 2011; Satoh et al., 2013; Wagner et al., 2019). There are small producers of yacón in Argentina, specifically in the provinces of Jujuy and Salta (Manrique et al., 2005). They have found horticultural and medicinal uses for this species by commercializing the tubers.
The objective of the research was to evaluate the microbial activity of soils cultivated with yacón and inoculated with microorganisms which favor plant growth.
MATERIALS AND METHODS
This study was based on three trials of yacón crops during three years at places in the Central Valley of the Province of Catamarca, located in the semi-arid region of the northwest of the Argentine Republic. Sandy loam was the soil textural class of the sites were Yacón was cultivated (sand: 73.85; clay: 8%; silt: 18.2%; pH: 7.5; M.O.: 5.72%).
In the plantations, propagules of yacón (Smallanthus sonchifolius) of around 30 grams each and the native soil microorganisms were used. The microorganisms were Azospirillum brasilense and mycorrhizal fungi, obtained from colonized forage species that acted as trap cultures (Di Barbaro et al., 2018).
An experimental design of randomized blocks with three repetitions per treatment was established in each trial. Each repetition corresponded to a 3 x 3m plot with 25 plants (experimental units), in 5 cultivation lines of 70 cm apart. The microbial inoculation treatments applied on the yacón propagules at the time of planting were: T0: Control or control (not inoculated); T1: Inoculation with A. brasilense; T2: Inoculation with native mycorrhizal fungi, and T3: Joint inoculation with A. brasilense and native mycorrhizal fungi.
As far as the inoculated treatments, the selected microorganisms were applied to the yacón propagules by sinking them in the inoculant just before plantation. In the meantime, the propagules of the control treatments were placed in sterile running water.
The native strain Pi 8 of Azospirillum brasilense was used, isolated from the endorhizosphere of paprika (Capsicum annum var. Elephant trunk) grown in the Province of Catamarca, and biochemically and molecularly identified (Tarrand et al., 1978; Döbereiner et al., 1995; Caballero-Mellado, 2002). The concentration of A. brasilense used for the inoculations was of 5x107 azospirillum. mL-1 quantified in a Neubauer chamber (Manacorda et al., 2007).
The inoculum of mycorrhizal fungi native to the province consisted of roots of Melilotus officinalis L., Avena sativa L., Hordeum vulgare L., Secale cereale L., Panicum maximum Jacq. and Cenchrus ciliaris L. colonized by these. The percentage of mycorrhizal colonization of the roots used as inoculum was 81.38%, estimated by the method of line intersections and microscopic observation of roots by Sieverding (1983) and McGonigle et al. (1990).
Soil samples were collected before planting the crop (T0) and at the time of harvest. The latter were collected from the rhizosphere of the plants using the set-up treatments. The collected samples were refrigerated at 4°C and transferred to the Laboratory of Agricultural Microbiology of the National University of Catamarca in order to determine the Total Biological Activity by hydrolysis of fluorescein diacetate (FDA).
The samples at the beginning of cultivation (T0) were taken between the months of October and November. Those at the end of the harvest were taken between May and July of each evaluated year. The evaluations of this research were carried out for 3 consecutive years, from 2017 to 2019, in different batches of production.
The biological activity was determined by the fluorescein diacetate hydrolysis (FDA) method according to Schnürer and Roswall (1982). Quickly, 5 g of the soil to be evaluated and 20 ml of potassium phosphate buffer solution 60mM (pH 7.6) are added in an Erlenmeyer flask. Then, 0.2ml of stock solution of FDA (2 mg / ml of acetone) is added and incubated for 20 minutes with stirring (200 rpm) at 25°C. The reaction is then stopped by adding 20ml of acetone to the Erlenmeyer flask, filtered (Whatman filter paper # 1) and read in a spectrophotometer at an absorbance of 490nm (Figure 1).
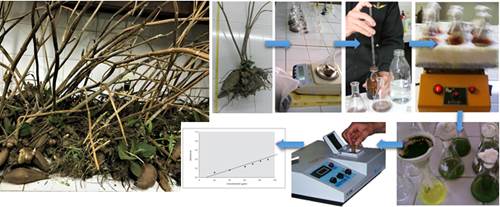
Figure 1. Procedure for the analysis of the total biological activity by hydrolysis of fluorescein diacetate.
To obtain the FDA concentration, the standard curve was previously determined. In order to do this, solutions of concentration of known FDA (0µg, 100µg, 200µg, 300µg, and 400µg) were prepared and subjected to complete hydrolysis. In measurements with the spectrophotometer, the relationship between absorbance (A°) and concentration (C) of FDA was obtained. Then, the absorbance data obtained from the different soil samples taken from the crops were adjusted according to the standard curve.
The results were analyzed by analysis of variance (ANOVA) and the means were compared by means of Tukey's test at a significance level of α ≤ 0.05. Statistical analyzes were performed with the Infostat statistical program (Di Rienzo et al., 2018).
RESULTS AND DISCUSSION
The results of this research indicate that the biological activity of soils was influenced by microbial inoculation. The highest global enzymatic activity, measured as FDA hydrolysis, recorded statistical differences when microorganisms that promote plant growth were applied (Table 1).
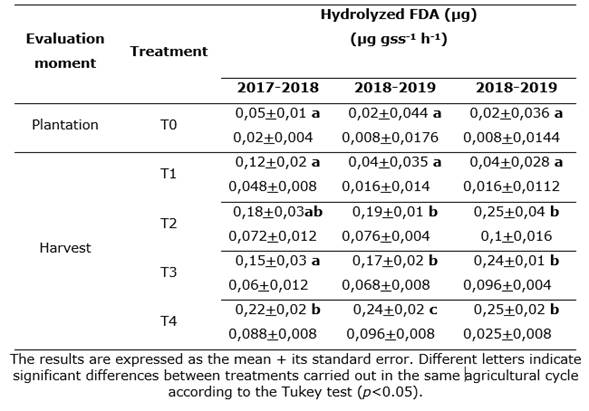
Table 1. Hydrolysis of ADF (μg; μg gss-1 h-1) in soils cultivated with yacón in three cultivation cycles in the province of Catamarca.
The highest TBA by ADF hydrolysis was recorded in soils cultivated with yacón inoculated with the microbial consortium of A. brasilense and mycorrhizal fungi. Statistically significant differences were obtained (p<0.05) between the TBA values of T3 in relation to the soil samples from the moment before crop plantation and the soil samples cultivated with yacón corresponding to the control treatments without inoculation microbial (T0) (Table 1).
The soil cultivated with yacón created differences in biological activity, recording the highest TBA values at harvest, while the lowest TBA values were determined prior to yacón plantation (T0). The results match those obtained in soils cultivated with topinambur (Helianthus tuberosus) (Di Barbaro et al., 2020).
When comparing the four treatments applied to the yacón culture, statistically significant differences between the treatments with the inoculation of the microbial consortium (A. brasilense and mycorrhizal fungi or T3) and the control or non-inoculated control (T0) were observed.
The greater biological activity observed with the microbial co-inoculation (T3) is explained because when the mycorrhiza is formed, the radical physiology and the exudates that these generate are altered and located in the soil associated with the yacón roots. Consequently, the surrounding microbial population is changed. This contributes to the nutrition of the plant as it explores a larger volume of soil and increases the microbial activity (Blanco and Salas, 1997; Brundrett, 2009). On the other hand, Azospirillum is a bacterial genus widely known for its ability to promote plant growth; its promoter substances stimulate root growth, which increases its nutrients and water absorption potential, key benefit for crops grown in arid and semi-arid regions (Bashan et al., 2007).
In treatments inoculated with A. brasilense (T1) and with mycorrhizal fungi (T2), a higher TBA was obtained as compared with treatments without inoculation (T0). However, the differences were not always statistically significant (Table 1). These results in lots grown with yacón crops are similar to those obtained in soils grown with topinambur crops (Di Barbaro et al., 2020).
Therefore, improving the quality of the microflora of agricultural soils from the incorporation of organisms selected for their contribution to the production and development of crops is an alternative that would result in better crops, increased yields, and soil fertility (Caballero-Mellado, 2002).
One of the environmental variables that influences the biological activity of the soil the most is temperature (Rodríguez-Gutiérrez, 2014). This variable increases the microbial growth in the mesophilic range (between 15 and 45ºC, with an optimum range between 30 and 40ºC), which is basically recorded during spring and summer seasons. However, the TBA of the soil samples at the time of harvest, taken during the months of the lowest temperatures in the Central Valley of the Province of Catamarca (late autumn and early winter), were higher than those recorded before planting during spring, mainly in the treatments with microbial inoculation (Table 1).
Long summers with high temperatures and short, cool, dry winters characterize the Central Valley of Catamarca. Throughout the year, the average temperature generally varies from 6 to 33°C (https://es.weatherspark.com/y/27905/Clima-promedio-en-San-Fernando-del-Valle-de-Catamarca-Argentina-during-all-the-a%C3%B1o).
The TBA of the soils before planting the yacón crop (T0) in spring and the TBA at the end of the crop (T0) in autumn / winter are similar despite the difference in temperatures in each season. For this reason, it is believed that the effect of the yacón cultivation resulted in increased microbial activity to levels comparable and slightly higher to those recorded during spring, before plantation (Table 1). This may be due to the interactions occurring in the rhizosphere and the higher root growth that create greater enzymatic activity and elevate the content of organic matter.
The vegetation, as well as the exudates produced by some roots, change the physical and chemical properties of the soils associated with the roots. These specifically include the structure, the porosity, the pH, and the redox potential, factors that together influence the density and activity of the microorganisms (IGAC, 1993; Sánchez de Prager, 2007). This explains the higher biological activity at the end of the crop despite the fact that the evaluation was carried out in late autumn and early winter, when the soil temperature is low (<7ºC) and the microbial populations decrease. Soil temperature influences root exudation which affects rhizosphere conditions. Outside the optimal range (25 - 35ºC), permeability is altered and metabolism and exudation are reduced (Sánchez de Prager, 2007), altering the biological activity of the soil.
The values obtained revealed a positive effect on the soil enzymes activity, explaining the results observed in this work. What is more, they reveal that, in most of soils, the microorganisms dominate their biological component and respond quickly to environmental changes (Sánchez de Prager, 2007; Rivero-Herrada et al., 2016; Gómez-Fernández et al., 2017). They are essential in the multiple functions of the soil; they are part of almost every known metabolic reaction and constitute the driving forces that supply energy and nutrients (Paolini-Gómez, 2017).
CONCLUSION
The inoculation of the yacón crop (Smallanthus sonchifolius) with the microbial consortium of A. brasilense and mycorrhizal fungi increase the TBA of soils associated to the yacón roots. This represents an agricultural management strategy destined to improve the fertility of cultivated soils.














