INTRODUCTION
The seed is an essential structure for the preservation of the species. During its maturation, it loses water to enter a stage of temporal latency. In this stage, it can remain days or years until it finds favorable conditions to initiate the process of imbibition with the absorption of water, propitiating the development of the embryo and the beginning of germination.
Organic compounds such as lipids, carbohydrates, proteins, and other compounds that are consumed during germination are stored in the seed. These reserves should be enough until the leaves initiate the photosynthesis from which the plants become autonomous (Ibrahim, 2016). These reserves depend on the type and evolution of plants (Ahmad et al., 2017). In species cultivated as grains with high concentrations of oils, lipids are the main reserve of the embryo, but for others such as corn and wheat, proteins and starches are the ones that serve as reserve (Ibrahim, 2016). In conifers and other neartic plants, the seeds greatly reduce their water content, while in the neotropics, this seed is an important element. It is then foreseeable that, in the process of germination, with the absorption of water in the process of imbibition, compounds of different types can be released into the surrounding environment.
Depending on the type of seed, exudates and production areas are different. These compounds are typically found as part of the cellular components and the metabolic process of the plant: sugars such as sucrose, raffinose, stachyose, and verbascosa; also, amino acids, organic acids, flavonoids, steroids, and proteins, volatiles compounds such as aldehyde, alcohol, ethylene, Co2 and volatile fatty acids (Long et al., 2015; Krishnappa et al., 2017). The release of this type of compound can be measured, altering the germination condition of the seed by modifying the pH (acidifying). This process causes the release of exudates, which have biochemical effects that can be evaluated (Sánchez-Pérez et al., 2010; Ibrahim, 2016).
Another important factor in germination is hormonal regulation since it controls and coordinates cell division and differentiation. Hormones such as abscisic acid (ABA), ethylene, gibberellins, auxins (IAA), phytocyanin’s among others are substances that control the physiological and biochemical processes of plants. In these cases, the Indole-3-acetaldoxime (IAOx) can transform into Indole glucosinolate (IG) and phytoalexins that intervene in the plant’s defense system (Duca et al., 2014; Tabatabaei et al., 2016).
Tryptophan is an amino acid produced in plants from which several metabolic pathways arise; these lead to the synthesis of plant hormones such as Indoleacetic acid (IAA). The substances participating in the IAA synthesis pathways in plants have been described as TRP-dependent (TRP-D) and TRP-independent (TRP-I) (Hernández-Mendoza et al., 2008, Gao et al., 2016, Lehmann et al., 2017).
TRP-D pathways have been well studied and described in both plants and microorganisms. These include the IAM pathway, the IPA pathway, the TAM pathway and the indole-3-acetaldoxine (IAOX) pathway (Aguilar-Piedras et al, 2008; Hernández-Mendoza et al, 2010; Mano and Nemoto, 2012; Korasick et al, 2013, Yang et al, 2007; Cook et al, 2016, Lehmann et al, 2017).
The presence of a TRP-I pathway is practically unknown in plants. For TRP-I, tree ways are reported. From KYN, Chorismic acid or Glutaraldehyde 3 phosphate (Berstad et al, 2014; Anjum et al, 2015; Mironova et al, 2017; Uribe Bueno et al, 2020). This is the KYN pathway; it derives from the TRP to the formation of kynurenic acid, anthranilic acid, or 3-hydroxykynurenine. The metabolites of this group are associated to cofactors, neurotoxins, carcinogens, toxins, and plant-growth promoters (Katoh, et al, 2006). In maize and other plant species, the TRP-I pathways are considered the main routes of IAA synthesis (Berstad et al, 2014; Mironova et al, 2017) as well as in microorganisms (Katoh et al, 2006). KYN can lead into other metabolic pathways, such as quinolinic acid in animals (Heyes et al, 1997; Pawlak et al, 2003; Chang et al, 2003; Korasick et al, 2015; Sipahi et al, 2016).
In TRP-I, the AA may be exogenous and formed from chorismic acid, thereby, entering the IAA synthesis pathway. From AA, it passes to Indole-3-glycerol phosphate (I3GP) (Cohen et al., 2003), which is transformed into indole, and hence it can be synthesized into TRP itself or IAA (Aguilar-Piedras et al, 2008; Chang et al, 2003; Cohen et al, 2003; Hernández-Mendoza et al, 2010; Korasick et al, 2015).
KYN is a metabolite whose presence is reported in animals (including humans), plants, and in the case of salmon, it is considered a pheromone. In plants, KYN is an important metabolite, which is produced during the catabolism of TRP in an indirect pathway to the synthesis of indole acetic acid and quinolinic acid (Katoh et al, 2006; Berstad et al, 2014; Badawy, 2017). KYN has been detected in germinating corn seeds (Zea mayz cv Golden Bantam), where it is considered a precursor in the formation of niacin and of aspartate and NAD in Arabidopsis thaliana (Katoh et al., 2006). The presence of NAD has been reported in Pea (Pisum sativum) when studying the degradation of TRP from which tryptamine, acetaldehyde, and indole acetaldehyde forms before synthesizing the IAA (Moore and Shaner, 1968; Quittenden et al, 2009). In other cases, after the decarboxylation that is carried out after the formation of anthranilic acid, it can be transformed into aniline, which is usually synthesized from the nitrification of benzene, and it is used to prepare compounds totally unrelated to plants (Hamdy et al, 2012).
In bacteria, such as Azospirillum brasilense, KYN is cleaved into anthranilic acid (AA), which is transformed into 1-(2carboxyphenylamine)-1deoxi-D-ribulose-5-phosphate and then into 3-indole glycerol phosphate to then synthesize into IAA finally. At this point, the AA can come. In addition to coming from KYN from chorismic acid (Hernández-Mendoza et al, 2010), the cycle can be restarted because after the formation of indole, L-TRP can be newly synthesized with the incorporation of L-Serine (Parthasarathy et al, 2018).
In A. brasilense four metabolic pathways in the degradation of TRP for the IAA synthesis have been reported: all of them known as TRP-dependent. These pathways are, Tryptamine (TRM), Indole Acetonitrile (IAN), Indole Acetamide (IAM), and Indole 3 pyruvic acid (IPyA). From the pathways mentioned above, the most important for agricultural purposes is undoubtedly the IAN pathway, as A. brasilense strains that produce the nitrilase enzyme when synthesizing the IAA, release a nitrogen molecule, which is why nitrogen fixation to the soil is attributed to the bacteria (Aguilar-Piedras et al, 2008; Yue et al, 2014).
The presence has also been detected in plants such as maize, where it has been shown that its production decreases with age and disappears 35 days after the seed has germinated (Singh and Widholm, 1975). The AA has also been reported to induce plant growth in several species such as rice (Wakasa et al, 2006) and work as a pheromone in insects (Arakaki et al, 2003). Also, in A. brasilense, the AA can be transformed into Indole-3-glycerol phosphate, from which the metabolites can follow two paths. One from IAA, the synthesis pathway from chorismic acid is currently reported, passing then to AA, which finally transforms into TRP (Aguilar-Piedras et al, 2008; Prombunchachai et al, 2017; Pavlova et al, 2017).
In fungi, specifically in T. atroviridae, T. viridae and T. harzianum, there are reports of synthesis of IAA and other auxinic substances, and there is no information on the metabolic pathways or the mechanisms employed by the fungi by themselves in association with plants for the synthesis of the metabolite (Gravel et al, 2007; Duca et al, 2014).
The production of IAA is considered relevant since in conjunction with Indole butyric acid, they are the main auxinic type and vegetal hormones that are produced by diazotrophic organisms such as A. brasilense (Aguilar-Piedras et al, 2008). In fact, IAA is a direct precursor of AIB, which is formed by passing through the microsomal membrane in maize cells using acetyl-CoA and ATP as co-factors. This phenomenon has also been observed in other plants (Ludwing-Muller, 2000).
This paper reports the results obtained through HPLC analysis performed in vitro to detect the presence of metabolites in the transformation routes from tryptophan into indole acetic acid in germinating seeds of nine cultivated plant species.
MATERIALS AND METHODS
The experiment was performed under laboratory conditions. The seeds were purchased from commercial seeds distributors. A completely random design was used with six different seeds and six repetitions of each of them. The parameters are recorded every 24h until 96hr. To meet the purpose, the seeds mentioned in Table 1 were placed in a small Petri dish (5mm) with 6 ml of sterile deionized water. At the end, the seeds were dried in an oven (80°C for 24hrs.). Subsequently, they were weighed, and a radicle test, cotyledon, and total weights were quantified. The weights were then compared with the dry weight that the seeds had before starting the experiment (Hernandez-Mendoza et al, 2012).
Table 1. List of seeds used in the study, acquired in the commercial seed market and pH values recorded during the experiment.
| Seed | Variety | Hours | ||||
|---|---|---|---|---|---|---|
| 0 | 24 | 48 | 72 | 96 | ||
| Sorghum bicolor | Patron | 6.2 | 6.2 | 6.2 | 6.4 | 6.6 |
| T aesativum | Salamanca S75 | 6.2 | 5.4 | 5.7 | 5.9 | 5.3 |
| Zea mayz | 30P49 | 6.2 | 6.7 | 7.0 | 6.8 | 6.8 |
| Phaseolus vulgaris | Negro Michigan | 6.2 | 5.8 | 5.6 | 5.7 | 7.3 |
| G hirsutum | Fiber Max | 6.2 | 6.0 | 5.9 | 6.6 | 7.0 |
| Cucurbita maxima | Gigantes | 6.4 | 6.3 | 6.1 | 6.5 | 6.5 |
With the previous data, a variance analysis with the Tukey test was performed (DMSH, p<0,05) (GraphPad Software, 2005) to find out the biomass distribution at the end of the experiments.
As for the leachates, the 96hr samples were analyzed separately after inoculation, recovering one milliliter of them in each replication. Subsequently, they were mixed in order to obtain three samples per species and stored at -20ºC until their analysis. The leachates were thawed at room temperature, filtered with 0.45 μm nylon membranes (Millipore™; Cork, Ireland) and collected in 1.5mL vials (National Scientific™, Rockwood, USA) (Hernandez-Mendoza et al, 2012).
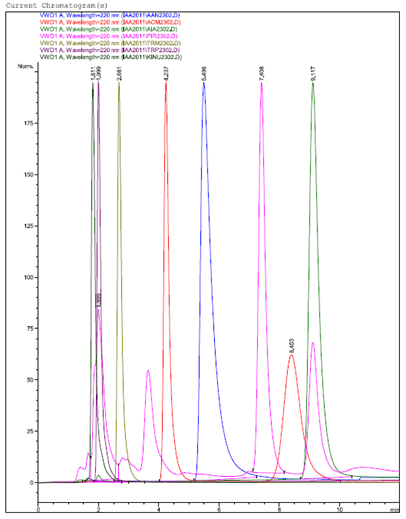
The samples were injected into a HPLC (Hewlett Packard-Agilent™ model 1100; Waldbronn; Germany) and run on a mobile phase 80/20 (800 mL of acetonitrile and 200 mL of water) with a pH of 3, a flow of 1 mL/min, in a C18 ultrasphere column of 150*4.6 MM (Beckman Ultrasphere™; Fullerton, USA). A detector was used (UV G1314A; Hewlett-Packard Agilent™) at a wavelength of 220 nm (Hernández-Mendoza et al, 2012). The information obtained was compared against pure commercial products of the following metabolites: tryptophan (TRP, Sigma-Aldrich), 3 indole acetic acid (IAA, Fluka™), 3 indole pyruvic acid (IPA, Sigma™), tryptamine (TRM, Sigma™), indole 3 acetamide (IAM, Aldrich™), 3 indole acetonitrile (IAN, Aldrich™), anthranilic acid (AA, Aldrich™), and L-kynurenine (KYN, Aldrich™). The chromatogram of the respective standards used is show in Figure 1. All results were analyzed with the SAS statistical package (SAS, 2008).
RESULTS AND DISCUSSION
The seeds of all studied species release compounds into the environment where they germinate. This action transforms into an important decrease in pH values. This can happen when using deionized water, and in extreme cases, the pH can go from 6.2 to 5.4 in 48 hours, as it does in wheat. In the case of tap water, which has a pH of 8.8, it can reach 5.4 at 72 hours after the trial has started (no showed data). The decrease in pH presents itself during and after the first hours, and this factor gradually increases until it exceeds the initial values (Table 2). Apparently, this is a natural phenomenon that could facilitate seed germination by solubilizing the nutrients found in the surrounding microenvironment. The impact of the release of the compounds when the seed is in the ground is large since the particles are amalgamated around them to form a pellet which can reach up to 2 cm in diameter.
Table 2. pH registry of seeds of different plant species that were tested during the study.
| Seed | Variety | Hours | ||||
|---|---|---|---|---|---|---|
| 0 | 24 | 48 | 72 | 96 | ||
| Sorghum bicolor | Patron | 6.2 | 6.2 | 6.2 | 6.4 | 6.6 |
| T aesativum | Salamanca S75 | 6.2 | 5.4 | 5.7 | 5.9 | 5.3 |
| Zea mayz | 30P49 | 6.2 | 6.7 | 7.0 | 6.8 | 6.8 |
| Phaseolus vulgaris | Negro Michigan | 6.2 | 5.8 | 5.6 | 5.7 | 7.3 |
| G hirsutum | Fiber Max | 6.2 | 6.0 | 5.9 | 6.6 | 7.0 |
| Cucurbita maxima | Gigantes | 6.4 | 6.3 | 6.1 | 6.5 | 6.5 |
A phenomenon that occurs simultaneously is that when the seeds hydrate, they apparently increase in size when imbibing; fact that is reflected in the decrease in dry weight thereof. The wear is observed as a decrease in the pH of the surrounding medium and when the seed is dried, as in the case of sorghum, this loss reaches up to 20% of the total dry weight at 96 h after the initial tests.
The difference amongst the values of dry weight and the total corresponds to the weight loss that the seed has during the germination process. In some cases, it could be considered a minimal loss. Table 3 shows the values at the moment the test starts and dry seeds after 96 hrs. of treatment. In all cases, a little weight loss was observed. This weight loss is assumed to come as a result of the amount of compounds released during the process of imbibition and germination of the seeds. When performing an analysis of the information obtained from the new biomass distribution, in the case of black beans, the radicle formed at 96 hrs., which represents almost 23% of the total dry weight. In this case, not even the sum of the radicular biomass formed in the germination process is enough to compensate for the weight loss of the seed. It is the same case for wheat and sorghum, which had already begun the germination process, and their radicle was visible. The dried weight loss is obvious anyway. Therefore, the phenomenon observed shows a similar behavior amongst the mono and dicotyledonous species that were studied.
Table 3. Relationship between dry weight at the start of the study and the weight distribution of the different parts of the seed and the formation of new biomass produced by germination. N/A non appliance.
| Seed | Variety | Dry weight | Radicle weight | Cotyledon weight | Testa | Total |
|---|---|---|---|---|---|---|
| Sorghum bicolor | Patron | 0.24 | 0.02 | 0.173 | N/A | 0.19 |
| T. aesativum | Salamanca S75 | 0.22 | 0.01 | 0.18 | N/A | 0.19 |
| Zea mayz | 30P49 | 1.65 | N/A | 1.561 | N/A | 1.56 |
| Phaseolus vulgaris | Negro Michigan | 2.4 | 0.048 | 1.811 | 0.23 | 2.31 |
| G. hirsutum | Fiber Max | 0.77 | N/A | 0.613 | N/A | 0.61 |
| Cucurbita maxima | Gigantes | 0.37 | N/A | 0.34 | N/A | 0.34 |
The HPLC quantification of the auxinic compounds present in the exudates during germination (Table 4) showed significant differences (0.05) in bean seeds (P vulgaris) with respect to other species. In these, the highest concentrations of TRP, TRM, IAN, IAM, KYN, AA, and IAA were detected, with the exception of IAP, where the highest concentration of cotton swabs was detected (G hirsutum). These values may be related to the physiological characteristics of P vulgaris as natural fixatives of atmospheric nitrogen and the ability to associate with soil nitrogen-fixing microorganisms.
Table 4. Quantification ppm of plant growth-promoting substances identified in several plant species´ seeds during germination.
| Seed | Variety | TRP | IAP | TRM | IAN | IAM | KYN | AA | IAA |
|---|---|---|---|---|---|---|---|---|---|
| Sorghum bicolor | Patron | 3.57c | 3.80c | 1.18c | 3.69b | 0.53b | 6.21c | 0.44a | 0.91b |
| T aesativum | Salamanca S75 | 11.55b | 2.10d | 0.68c | 4.48b | 0.86b | 11.09b | 0.17b | 0.32c |
| Zea mayz | 30P49 | 1.94c | 1.16d | 0.60c | 3.54b | 0.50b | 3.92c | 0.15b | 0.22c |
| P vulgaris | Negro Michigan | 16.47a | 5.57b | 7.04a | 18.12a | 2.58a | 40.91a | 0.50a | 2.05a |
| G hirsutum | Fiber Max | 2.99c | 22.98a | 1.82b | 4.16b | 0.64b | 4.97c | 0.18b | 0.38c |
| Cucurbita maxima | Gigantes | 2.73c | 1.42d | 0.90c | 2.38b | 0.77b | 6.95c | 0.09b | 0.92c |
In this study, the synthesis of KYN and AA is considered important, since it can come from an alternating TRP route and potentially transform into IAA. Plants use different routes in the homeostasis of the IAA. Besides, the route depends on environmental factors such as light, temperature, among others.
From the auxinic compounds analyzed in the exudates, the KYN has a greater concentration (Table 3). From this data, it can be estimated that it is a route of great activity between the analyzed species. Considering the IAA biosynthetic route dependent on the TRP precursor, high concentrations of IPA and IAN are observed in these results. Next in line of importance are TRM and IAM, reported as intermediate compounds in the catabolic pathways of TRP-D. As for the amino acid TRP as source of origin for the synthesis of the AIA, the seeds release it during impairment and the initiation of germination, among other components, and it is the main precursor for the biosynthesis of the microbial IAA.
On the other hand, the low concentrations of AA can be due to the fact that KYN is transformed into both AA and in other compounds that go towards the synthesis of quinolinic or picolinic acids, phenolic compounds, and molecules that constitute the starting materials for the biosynthetic routes of secondary metabolism.
The process of seed imbibition is essential for germination. In this process, a series of metabolic events are present within which compounds of different kinds are released to the surrounding environment (Rosental et al., 2014; Née et al., 2017). In this study, the auxinic compounds present in the germination exudates of the seeds were quantified through the HPLC method whether mono or dicotyledonous. In the same way, this method has been used in the quantification of auxinic compounds, product of the interaction between the plant and microorganisms (Haichar et al., 2014; Palacios et al., 2016).
During the process of imbibition, the analyzed seeds released exudates that acidified the surrounding medium. This was caused possibly by the disintegration of the cell membranes that propitiate the release of organic acids, sugars, and ions among which the H+ is, reflected in a pH decrease (Ávila et al., 2015; Pérez-Rodríguez et al., 2016). This is associated with the nutrient absorption mechanism as this condition makes the nutrients present available, facilitating plant growth processes (Tsunoda and Van Dam, 2017; Van and Bouwmeester, 2016).
Imbibition is a process of acquiring water through the cotyledons of the seed, thus activating the mechanisms of germination of the seed (Yue et al., 2014; Shu et al., 2016). These moments are characterized by a visible enlargement of the seed (Domínguez et al., 2007). However, during this enlargement, there is also a release of compounds in the surrounding environment, and this whole process at the end brings with it a decrease in the dry weight of the seed (Rosental et al., 2014). In this study, all the seeds analyzed show the afore-mentioned weight reduction at the end of the test, although some of them have already begun the process of germination and formation of new plant matter.
Out of all the auxinic compounds that were analyzed, the KYN is the substance that is detected with higher values, a phenomenon that had not been previously reported since the works mention only the presence of the metabolite in fish, insects, humans, and rats (Berstad et al., 2014; Anjum et al, 2015; Mironova et al., 2017). The detection of the AA shows the possibility that the route of TRP-I is active among the plant species analyzed, although this metabolite may have one origin or two. One from the KYN (Vega et al., 2016; Palacios et al., 2016) and at the end of the TRP. The other with the participation of the anthranilate synthase from chorismic acid (Chang et al., 2003; Hernández-Mendoza et al., 2012). This metabolite may also be synthetized from chorismic-shikimic acid; however, other studies analyzing the IAA synthesis pathways do not incorporate this substance within the TRP to IAA transformation routes in plants (Lwdwig-Muller, 2000; Aguilar-Piedras et al., 2008; Quittenden et al., 2009). The IAA presence was detected previously in other experiments in maize (Hernández-Mendoza et al., 2010) and bean seeds (Hernández-Mendoza et al., 2012).
The germination process begins with the absorption of water by the seed, which may be 200 µL in the first 24 hours, as in the case of maize. This is smaller in the following hours (Hernández Mendoza et al., 2010). The volume is consistent with the size of the seeds evaluated in this study. When the available water does not meet the required quantities, the seed increases its size; however, this action stops the germination process. In this experiment the administered water was sufficient to ensure proper germination.
Imbibition brings in available water, so the cotyledons may start the release of germination-related compounds and bring a physical-chemical wear that consumes the reserves. That is why the seed loses weight when passed through the drying oven. This phenomenon has been previously reported (Hernández-Mendoza et al., 2012). According to the observed herein, some seed species lose more weight than others.
The pH is a relevant factor in the plant´s nutrient absorption since its values may facilitate or impede the nutrients from entering, even if they are found in the soil and in non-deficient amounts. In the moment of germination, the release of compounds has a modifying impact in the environment of the seed (2mm diameter average in the DK54 sorghum), which is larger than 1 cm (Figure 2. Hernández-Mendoza, not published). In a generalized manner, an increase and then a decrease in pH values was observed. The latter occurs when the actual germination of the seed takes place.
The pH values have great importance in the pathogenic processes of fungi. On example is the expression of the monooxygenase gene in T. hamatum, which plays an important role in the antagonistic activity; the levels of expression can be modified by the pH and the presence of phytopathogens such as Sclerotinia sclerotiorum, S. minor and Sclerotium cepivorum (Carpenter et al., 2008).
The importance of the production of enzymes such as monooxygenase is based on the fact that when Agrobacterium tumefasciens is detected along with cytokinins, the infectious process caused by that bacterium in plants is developed (Kobayashi et al, 1995).
An alternate pathway that has been described in bacteria such as A. brasilense is the Kynurenine pathway that transforms into IAA through AA (Hernández-Mendoza et al, 2010). The Kynurenine is present in species such as oats, corn, bean, safflower, and soybean, and other studies have reported it (Hernández-Mendoza et al., 2010).
AA, one of the KYN derivatives, was detected in this study in P. vulgaris var Pinto Saltillo and in safflower. This has been previously reported in corn and bean as well as in the supernatant where the A. brasilense bacterium was cultured (Hernández-Mendoza et al., 2008; Hernández-Mendoza et al., 2012).
In the case of AA, very low values were detected. Besides, the possibility that the Kyn is transformed into compounds that go to other pathways such as quinolinic acid, picolinic, aniline, or to transporters of hydrogen ions can be considered (Nadh+).
One reason why the IAA concentrations are low is that it is a compound that can transform into very similar products when an amino acid is added or when reacting to form indole butyric acid or chloroindole acetic acid, among which there is a constant transformation that prevents the accumulation in tissues or in the medium (Kazan and Manners, 2009). This may be one of the reasons why there is no concordance in the quantity of KYN formed, the IAA concentrations, and the TRP in this study. In fact, the levels of the latter in the plant are restricted in a complex form.
CONCLUSIONS
The results obtained show that when initiating the process of imbibition, the seeds release compounds, thus impacting the decrease in the pH medium. Because of this, the seed loses dry weight although it has already begun the process of germination and formation of new biomass. Also, during imbibition, the seed releases tryptophan, one of the main precursors in the synthesis of indole acetic acid. In this study, the presence of auxinic compounds involved in the pathways Tryptophan-Dependent and Tryptophan-Independent were also analyzed. From TRP-D, there is indole-3-acetamide (IAM), 3-indoleacetonitrile (IAN), tryptamine (TRM) and they use this amino acid as a precursor. Anthranilic acid (AA) and kynurenine (KYN), which are part of the Independent TRP pathway, were also detected. The above compounds in addition to AIA and TRP were also detected in the study. The seeds lose weight and wear away during the germination process to form new biomass.