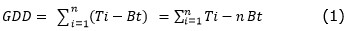INTRODUCTION
The feijoa, or pineapple guava (Acca sellowiana [O. Berg] Burret), is a plant native to South America, whose commercial production is important in New Zealand, Colombia, Azerbaijan, Georgia, California, and, recently, Brazil (Parra & Fischer, 2013). In Colombia, feijoa is produced adequately throughout the year in regions between 1,800 and 2,700 m above sea level (m asl), in orchards with different varieties, which guarantees cross-pollination and fruit quality at harvest (Parra-Coronado et al., 2019; Fischer et al., 2020).
The quality of fruits is influenced by the presence of organic acids and soluble sugars, as well as by changes in color, texture, and flavor, that contribute to the organoleptic quality of fruits (Bae et al., 2014). As the fruit develops, biochemical changes occur, mainly ethylene production, respiratory intensity, sugar and organic acid content, and polygalacturonase activity, among others (Balaguera-López & Herrera, 2012). In the early stages of development, fruits accumulate organic acids and, therefore, have an acid taste (Shiratake & Martinoia, 2007).
Three-quarters of the dry weight (DW) of woody plants comprise soluble carbohydrates, which are the main storage compounds and are organic substances from which most other organic compounds are synthesized (Pallardy, 2008), mainly with respiratory metabolism (glycolysis and Krebs cycle). In general, carbohydrates are very important because of their contribution to the texture, color, flavor, and nutritional value of fruits (Yahia et al., 2019). During the development and ripening of fruits, the sugars stored in vacuoles (Yahia et al., 2019) generally increase in concentration along with a simultaneous decrease in organic acids (Vallarino & Osorio, 2019). Sugars and organic acids play an important role in metabolic processes, being the main substrates involved in respiration and ripening, affecting the flavor and quality of fruits (Herrera, 2012).
The carbohydrates that accumulate during fruit development depend, among other factors, on environmental conditions: first of all, light and temperature, which greatly influence the content of carbohydrates produced in photosynthesis (Fischer et al., 2016a). Yahia et al. (2019) reported that feijoa contains 6.6g/100g FW (fresh weight) of total sugars, which is relatively low compared to other fruits, such as grapes (15.0g), mangoes (14.0g) or apples (14.0g), but greater than in the guava (5.7g). In the case of organic acids, Vallarino & Osorio (2019) confirmed that the processes involved in metabolism and accumulation in the mesocarp cells of fruits are under genetic and environmental control.
During the development of feijoa fruits, changes in their physicochemical traits are manifested, even among the fruits of the same cultivar, which are directly related to altitude and, therefore, climatic conditions (Parra-Coronado et al., 2017). The variation of physicochemical traits in feijoa fruits such as size, weight, total soluble solids, total titratable acidity, and firmness, is mainly influenced by altitude, temperature (in terms of thermal time), and precipitation recorded during the fruit development period (Parra-Coronado et al., 2015b; Parra-Coronado et al., 2016; Parra-Coronado et al., 2017).
Solarte et al. (2014) studied the influence of altitude on fruit quality in four guava genotypes grown in three municipalities (locations) of the Department of Santander (Colombia), finding an interaction between the genotype and the environment, which was significant for the content of organic acids (citric, malic, oxalic, succinic and ascorbic) and sucrose and fructose. They also found that, regardless of the genotype, the most abundant sugars were fructose (51%), glucose (26%), and sucrose (22%) in that order. In general terms, the sucrose content decreases gradually during the ripening process and depends on the environmental conditions of the production area and the genotype, while the glucose and fructose contents are not affected by the development stage or by environmental conditions (Solarte et al., 2014).
For variations of the organic acids in guava fruits, Solarte et al. (2014) reported that citric and succinic acids predominated in the different genotypes, with lower contents of malic and oxalic. They also indicated that, during the ripening process, there was a decrease in the contents of all organic acids, except ascorbic acid, and that these contents were influenced by the development stage and by the climatic conditions. In the case of some genotypes, they observed that the fruits produced at higher altitudes had higher contents of citric, ascorbic, malic, and oxalic acids.
Rodríguez et al. (2006) evaluated variations in contents of fructose, glucose, and sucrose in the last stage of development (growth) of feijoa fruits of clones 8-4 and 41 (Quimba), cultivated in the municipality of La Vega (Cundinamarca), finding that the most abundant sugars were fructose, sucrose, and glucose although there was very little variation from day 112 after anthesis to physiological maturity. At physiological maturity, the amounts of fructose, sucrose, and glucose in clone Quimba were respectively 4.1, 3.3, and 1.7%, while in clone 8-4, they were 4.9, 3.5, and 3.0%.
In the case of feijoa, there are no known studies that indicate variations in the content of sugars and organic acids during fruit development as a function of altitude and therefore climatic conditions, which vary with altitude. The objective of this study was to determine variations in the content of soluble sugars and organic acids of feijoa fruits of clone 41 (Quimba) during development in two production sites located at different altitudes.
MATERIALS AND METHODS
The study was conducted in two localities in the municipalities of Tenjo (locality 1) and San Francisco de Sales (locality 2) in the Department of Cundinamarca (Colombia), on plantations of feijoa of clone 41 (Quimba), established in 2006. Flower buds were marked in 20 trees per locality, making an hourly record of the climatic conditions (temperature, relative humidity, precipitation, and radiation) from anthesis to harvest, with automatic meteorological stations, iMETOS ECO D2 (Pessl Instruments, Weiz, Austria). The geographical coordinates and the climatic conditions registered in each location during the study are presented in Table 1.
Table 1 Geographic coordinates and climatic conditions recorded in the two locations during the development of feijoa fruits, from anthesis to harvest.
| Environmental and physiological variables | Location | |
|---|---|---|
| Tenjo (Locality 1) | San Francisco de Sales (Locality 2) | |
| Coordinates | Latitude: 4°51’23” N Longitude: 74°6’33” W | Latitude: 4°57’57” N Longitude: 74°16’27” W |
| Altitude (m asl) | 2,580 | 1,800 |
| Mean temperature (°C) | 12.3 | 18.5 |
| Mean relative humidity (%) | 76.4 | 86.1 |
| Thermal time - GDD (°C) | 1,979 | 2,728 |
| Accumulated precipitation (mm) | 190 | 573 |
| Accumulated radiation (W m-2) | 12,303 | 7,814 |
The management of the orchards in the two locations was performed in the same way (fertilization, pruning without application of irrigation, etc.) to eliminate the effect of crop variables.
Determination of thermal time. To complete a certain phenological phase, crops require an accumulated amount of heat, called "Thermal Time or Growth Degree Days (GDD)". There are several methods for calculating thermal time, but the most widespread and the one used in this study consists of obtaining the average of the maximum and minimum daily temperatures, from which the base temperature (Bt) for said phenological phase is subtracted, and the sum of the values is calculated until completing the phenological period or phase (Matzarakis et al., 2007; Parra-Coronado et al., 2015a). The estimated Bt for the development of the feijoa fruit of clone 41 (Quimba) was 1.76°C (Parra-Coronado et al., 2015a), a value considered for the determination of GDD, which is calculated from equation 1 (Parra-Coronado et al., 2015a).
i
Sampling. At each location, one fruit was taken weekly at random from the middle third of each of the 20 trees, starting the sampling on days 99 and 138 after anthesis, (daa), for San Francisco de Sales (1,800 m asl) and Tenjo (2,580 m asl) respectively when the feijoa fruits presented an adequate size (minimum sample) to carry out the respective analyses of organic acids and sugars. From the fruits collected weekly, 10 fruits were randomly taken for the determination of individual weight and size (length and diameter), for which an analytical balance "Precisa XT220A" was used, with a capacity of 220g and a precision of 0.0001g (Precisa Instruments, Switzerland), along with a digital electronic caliper with a precision of 0.01mm, respectively. Five fruits were also taken, which were frozen at -80°C, for subsequent determination of the content of organic acids (oxalic, citric, and malic) and sugars (fructose, glucose, and sucrose), with three repetitions per test.
Determination of soluble sugars and organic acids. The fructose, sucrose, and glucose contents of the feijoa fruits were determined with HPLC chromatography (Waters, Milford, Massachusetts, USA), based on the methodology described by Pérez-Mora et al. (2018) and Solarte et al. (2014). A Phenomenex Ca++ column was used, with mobile phase: deionized double distilled water, and a refractive index detector.
The contents of citric, malic, and oxalic acids were determined according to Pérez-Mora et al. (2018) and Solarte et al. (2014) using HPLC chromatography (Waters, Milford, Massachusetts, USA), with a ROA organic acid H+ column, mobile phase: 5mM H2SO4 and a photodiode array detector at 207nm.
Statistical analysis. With the data, normality tests were performed with the Shapiro-Wilk test and the homogeneity of variances test with the Levene test. The variables that did not meet these assumptions (malic acid and sucrose) were subjected to √x transformation. Subsequently, the analysis of variance by F-test and the LSD mean comparison test (p≤0.05) were performed, while the comparison between localities was performed by using the t-student test (p≤0.05). The analyses were performed with SPSS v. 19.
RESULTS AND DISCUSSION
Climatic conditions during fruit development. Figure 1 shows the variation of the mean relative humidity (RH), precipitation, and radiation accumulated through the period of development of the feijoa fruits (expressed as thermal time-GDD) for locality 1 (Tenjo) and locality 2 (San Francisco de Sales) during the study.
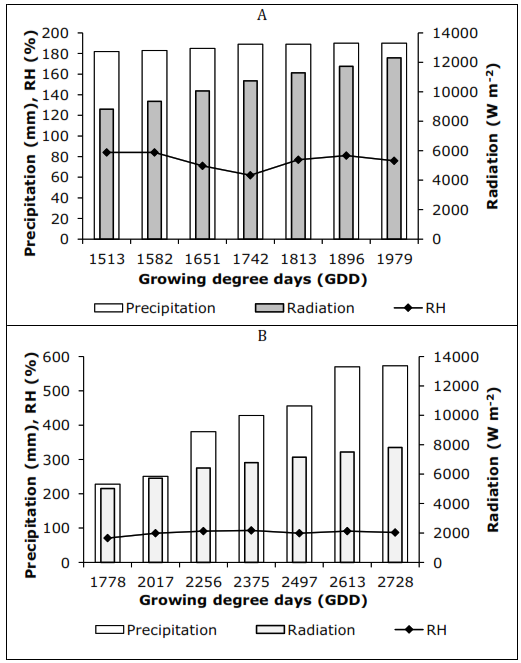
Figure 1 Behavior of accumulated precipitation, relative humidity (RH), and accumulated radiation at A) Tenjo and B) San Francisco.
It was observed that locality 1 (Tenjo) had a RH that varied between 62 and 84%, while the total accumulated precipitation varied from 182mm (1,513 GDD) to 190mm at the time of harvest with an accumulation of only 8mm during the development of the fruits characterized by a relatively dry period, which is consistent with the behavior of accumulated total radiation, which presented values from 8,816W m-2 (1,513 GDD) up to 12,303W m-2 (1,979 GDD) at harvest.
For locality 2 (San Francisco), RH was observed to vary between 71 and 93%, with total monthly accumulated precipitation ranging from 228mm (1,778 GDD) to 573 mm at harvest, with an accumulation of 345mm during fruit development, which differed greatly from the locality 1 (2,580 m asl), which was also observed in terms of the total accumulated radiation, which varied from 5,028 W m-2 (1,778 GDD) to 7,814 W m-2 at harvest. Locality 1 presented a drier climate, with less precipitation and higher total radiation than locality 2, at 1,800 m asl, during the development of the feijoa fruits. These conditions resulted in the development and fresh weight of the fruit at harvest of 155 days (2,728 GDD) and 38.1g in locality 2, as compared with 180 days (1,979 GDD) and 68.6g in locality 1 (Parra-Coronado et al., 2015b).
Organic acids. It was found that among the acids analyzed, the oxalic acid content was always less than 0.05mg g-1 fresh weight (FW), generally not detected. Citric acid and malic acid are the main organic acids in feijoa fruits, as described by Shakya & Lal (2018), for most fruits. At the Tenjo location 2,580 m asl, it was observed that the predominant acid was citric (≈54%), as well as in San Francisco (≈61%) (Figure 2).
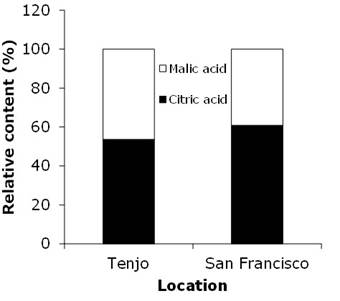
Figure 2 Relative contents of organic acids at harvest of feijoa fruits grown in two locations. Localities Tenjo and San Francisco.
Citric acid. The fruits that developed in locality 1 (Tenjo) presented statistical differences (p<0.05) in the acid contents through their state of maturation. It was observed that the highest content was in the fruits in physiological maturity, corresponding to fruits with 173 days after anthesis (daa) at 1,896 GDD (9.63mg g-1 FW) while at 1,513 GDD in green fruits (age 138) and 1,813 GDD in fruits at the beginning of maturation (age 166 daa), this acid presented the lowest value (6.13mg g-1 FW) (Figure 3A). In the San Francisco locality, with significant differences (p<0.05), the lowest acid concentration was obtained in green fruits (99), corresponding to the first sampling (1,778 GDD) with a value of 3.86mg g-1 FW. The concentration increased throughout growth and development, reaching the highest value at 2,613 GDD (mature state, 148 daa) with 16.35mg g-1 FW (Figure 3C). Irregular behavior was observed, with increases and reductions of the citric acid contents (Figure 3A and Figure 3C) with a tendency to increase during the development of fruits at the locality of 1,800 m asl, which confirms that feijoa fruits do not have a very sweet flavor (Yahia et al., 2019). This differs from the behavior of other Myrtaceae, as in the case of guava in the Santander region (Colombia), where citric acid decreased during fruit development (Solarte et al., 2014), or in Champa (Campomanesia lineatifolia) in Boyacá (Colombia), where this acid increased during fruit development (Balaguera-López & Herrera, 2012).
Figure 3 Contents of the organic acids, citric and malic, during the development of feijoa fruits grown in Tenjo (A and B) and San Francisco de Sales (C and D). Vertical bars in each mean indicate the standard error (n=3).
At harvest, the fruits at physiological maturity obtained at 1,800 m asl presented statistically higher contents of citric acid (Table 2), 79% higher than at 2,580 m asl. This result is opposite to that observed by Solarte et al. (2014) in guava, who found higher contents of this acid in the highest site (1,890 m asl), as compared to a lower one at 1,570 m asl. Similarly, Mayorga et al. (2020) measured a citric acid content about three times higher at a high altitude (2,498 m asl) in Pasca (Cundinamarca) in curuba (Passiflora tripartita var. Mollissima) than in the lower one (2,006 m asl). However, Fischer et al. (2000) did not find a difference in the citric acid content in the Cape gooseberry between two altitudes, 2,300 and 2,690 m asl, in the Department of Boyacá, which indicates that the content of organic acid is not a unified response to the different altitudes of fruit trees.
In the case of altitude, the concept of multidimensionality must be considered (Zandalinas et al., 2021), which is composed of climatic factors that, on the one hand, decrease with increasing altitude, such as temperature (0.6°C/100m) and the partial pressure of the air (CO2, O2 and N2) while radiation (especially UV), precipitation (from 1,500 m asl), and wind increase. Furthermore, these factors change constantly depending on the site (Fischer et al., 2020; Fischer & Ordúz-Rodríguez, 2012).
Malic acid. There were statistical differences (P <0.01) between the development stages of the fruits grown in locality 1 (Tenjo). During almost all development, malic acid was below 7mg g-1 FW, especially at 1,582 GDD (green fruit, 145 daa), where it presented the lowest value (2.64mg g-1 FW), but at 1,896 GDD (mature fruit, 173 daa), the fruits accumulated a large amount of this acid and reached a content of 13.24mg g-1 FW (Figure 3B). The fruits in locality 2 (San Francisco de Sales) also presented differential contents during their development (p<0.01), with a minimum value of 2.73mg g-1 FW at 1,778 GDD (green fruit, 99 daa ) and two important peaks: one at 2,017 GDD (with 9.84mg g-1 FW, green fruit, 113 daa) and the other with a greater accumulation at 2,613 GDD (10.10mg g-1 FW, fruit mature, 148 daa) (Figure 3D).
In the case of malic acid, it was not possible to establish a clear pattern of behavior in relation to the state of fruit development and altitude, as it was found, for example, in guava, in which this acid decreased during fruit development (Solarte et al., 2014). Likewise, the accumulation of malic and citric acids in the feijoa did not coincide with the general pattern of many fruits to accumulate the highest amount of organic acids in the early stages of fruit development (Shiratake & Martinoia, 2007). On the other hand, Solarte et al. (2014) indicated that at higher altitudes, the acid content is lower because of a constant decrease as fruit maturity advances. However, the non-existence of a statistical difference in the content of malic acid evaluated in the feijoa fruits at the two altitudes (Table 2) confirmed the findings of Fischer et al. (2000) for Cape gooseberry fruits, comparing sites at 2,300 and 2,690 m asl in Boyacá.
According to Parra-Coronado et al. (2015b), the total titratable acidity (TTA) presents a variable behavior and tends to increase slightly during the development of feijoa fruits, with values at harvest of 1.76±0.07% in locality 1 and 1.80±0.11% in locality 2, which agrees with the findings for citric and malic acids.
Table 2 Contents of organic acids and soluble sugars in the harvest of feijoa fruits grown in two locations.
| Location | Citric acid (mg g-1 FW) | Malic acid (mg g-1 FW) | Sucrose (mg g-1 FW) | Glucose (mg g-1 FW) | Fructose (mg g-1 FW) |
|---|---|---|---|---|---|
| Tenjo | 7.95±0.3 | 6.87±0.3 | 4.27±1.1 | 14.44±0.9 | 16.75±1.3 |
| San Francisco | 14.21±3.9 | 9.14±1.4 | 1.03±0.3 | 12.70±1.7 | 13.87±1.7 |
| Significance | * | ns | * | ns | ns |
Soluble sugars. In the two study locations, the fruits had higher fructose contents, closely followed by glucose and low sucrose contents. In locality 1 (Tenjo), fructose was 47.2%, and in locality 2 (San Francisco), it was 50.3% (Figure 4).
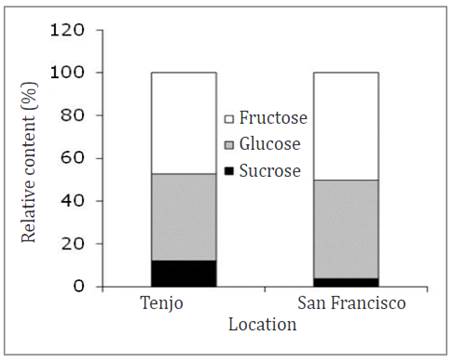
Figure 4 Relative content of soluble sugars at harvest of feijoa fruits grown in two locations: 1) Tenjo and 2) San Francisco.
Fructose. The content of this sugar changed significantly (p<0.01) during the development of the fruits grown in locality 1. The content was lower in the early stages of development (from 1,513 to 1,742 GDD, 138 to 159 daa), varying from 4.87 to 3.30mg g-1 FW while at 1,813 and 1,979 GDD (mature fruit, 166 to 180 daa), representative increases were observed, varying between 11.15 and 16.75mg g-1 FW (Figure 5A). In locality 2, with statistical differences (p<0.01), the fructose increased from 1,778 to 2,256 GDD (from 99 to 127 daa) and from 1.56 to 13.17mg g-1 FW, then decreased to 2,375 GDD (127 daa) to a value of 5.71mg g-1 FW, and finally increased until harvest (155 daa), where the highest content was obtained (13.87mg g-1 FW) (Figure 5D).
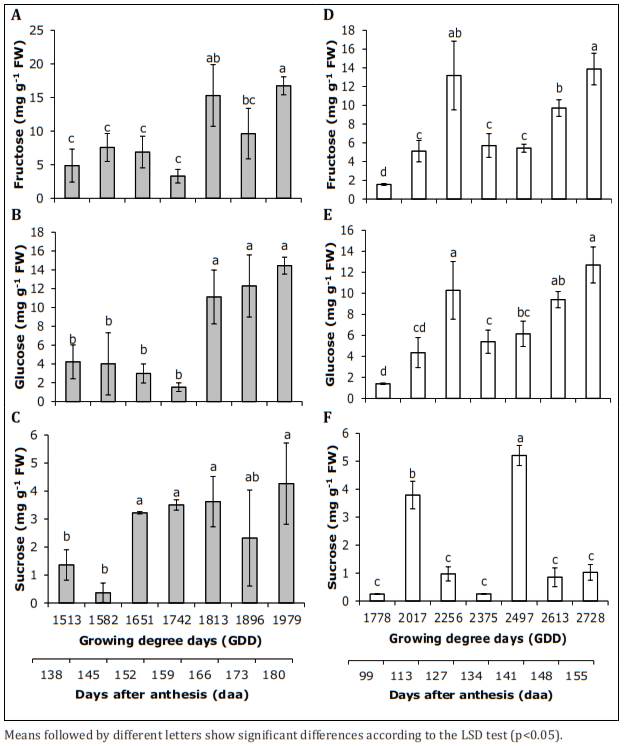
Figure 5 Contents of soluble sugars during the development of feijoa fruits grown in the location of Tenjo at 2,580 m asl (A, B, C) and San Francisco de Sales at 1,800 m asl (D, E, F).
The weather conditions of the two evaluated localities did not affect the fructose differently at harvest, presenting values of 16.75 and 13.87mg g-1 FW for Tenjo and San Francisco de Sales, respectively (Table 2).
The fructose content increased during the fruit development, which agrees with most fruits that accumulate monosaccharides, fructose, and glucose as a reserve for respiration (Lal, 2018). In addition, at the end of the fruit development, this sugar increases because of the degradation of sucrose to the monosaccharide fructose and glucose (Yahia et al., 2019). There was a tendency to accumulate more fructose at 2,580 m asl than at 1,800 m asl, so the authors assumed that, on the one hand, the incremented radiation at the highest site favored biosynthesis of this sugar because of an increase in photosynthesis (Yahia et al., 2019) or, on the other hand, the higher temperatures in location 2 contributed to a loss of this sugar because of fruit respiration (Taiz et al., 2017).
Glucose. In location 1 (Tenjo), the glucose contents were significantly (p<0.05) lower from 1,513 to 1,742 GDD (from 138 to 159 daa), varying between 4.22 and 1.53mg g-1 FW. Then, there was a representative increase that was maintained until harvest (180 daa); at this point, the content was 14.44mg g-1 FW (Figure 5B). In locality 2 (San Francisco de Sales), there were significant differences (p<0.01). The first two samplings (1,778 and 2,017 GDD, 99 and 113 daa) were characterized by lower contents of this sugar (1.39 and 4.35mg g-1 FW), then they had an increase to a value of 10.28mg g-1 (at 2,256 GDD, 127 daa), followed by a decrease. Subsequently, there was a gradual increase until harvest (155 daa), with a value of 12.70mg g-1 FW (Figure 5E).
The altitude also did not affect the content of this monosaccharide significantly (Table 2). Similarly, as in the case of fructose, glucose increased during fruit development, which is a normal behavior in almost all fruits (Herrera, 2012) because of the constant translocation of sucrose from the leaves to the fruits and its conversion into the two monosaccharides (Yahia et al., 2019). It was also observed that the contents of fructose and glucose hexoses were very similar, especially in the San Francisco locality, as seen in many species of fruit trees (Fischer et al., 2015).
Sucrose. For this sugar, there were some significant differences (p<0.05) throughout the fruit development in the Tenjo locality. The lowest contents of this sugar were obtained in the first stage of development, with a value of 0.37mg g-1 FW (at 1,582 GDD, green fruit, 145 daa); then, they increased to a value of 3.62mg g-1 FW (at 1,813 GDD, 166 daa). At harvest, the content was 4.27mg g-1 FW (Figure 5C). At 1,800 m asl, there were statistical differences (p<0.01); the contents in general were low (less than 1.00mg g-1 FW), except for 2,017 GDD (113 daa) and 2,497 GDD (141 daa), with a content of 3.79mg g-1 FW and 5.21mg g-1 FW, respectively (Figure 5F).
At harvest, there were differences in the sucrose contents when comparing the two localities. A higher content was evidenced in Tenjo, with 4.27mg g-1 FW. On the other hand, in San Francisco, the content was only 1.03mg g-1 FW (Table 2).
The increase in the sucrose concentration at 2,580 m asl during feijoa development showed a normal behavior in many fruits and showed a gradual translocation of sucrose that apparently occurred until the last moment of fruit development at this location, which could have been generated by the promotion of photosynthesis and the translocation of photoassimilates given the greater solar radiation of this locality (Figure 1) (Parra-Coronado et al., 2015b), as compared to the 1,800 m asl locality. In addition, the temperature at 1,800 m asl was 6.2°C higher than at 2,580 m asl (Table 1), which could have accelerated the degradation of this sugar through increased fruit respiration (Taiz et al., 2017), and confirmed the production of smaller fruits at this site (Parra-Coronado et al., 2015b). Conversely, the average temperature at 1,800 m asl, 18.5°C, was not the most suitable for the production of this crop, with 16°C reported as a favorable average temperature (Fischer & Parra-Coronado, 2020).
In general, the trend of soluble sugars analyzed during the development of the feijoa fruits agreed with the behavior of total soluble solids (TSS) found for these two locations by (Parra-Coronado et al. (2015b), Parra-Coronado et al., 2019), who found that TSS increased from 10.6±0.9 to 12.6±0.8ºBrix in Tenjo, and from 10.8±0.6 to 11.4±0.8ºBrix in San Francisco de Sales.
In general, the heterogeneous behavior, especially of the organic acids and, to a lesser extent, of the sugars, measured in the developing feijoa fruits could be explained by several reasons: (a) during harvest, different stages of development were used each time because they were destructive analyses even though fruits of the same age were marked from floral anthesis; (b) the incidence of light on the developing fruits varied depending on whether or not there were shading leaves (Fischer et al., 2012; Fischer et al., 2016b) because fruits under shade have decreased contents of organic acids (Vallarino and Osorio, 2019); and (c) higher or lower temperatures during fruit development could have an influence because organic acids react with a decrease when temperatures increase (Vallarino and Osorio, 2019; Fischer et al., 2016a).
CONCLUSIONS
In Tenjo, located at a higher altitude (2,580 m asl), where the solar radiation is higher and the temperature is lower, fruits were obtained at harvest maturity at 1,979 GDD, that is, 749 GDD less than in San Francisco de Sales, located at 1,800 m asl. The contents of organic acids accumulated in a greater proportion at 1,800 m asl, mainly citric acid; soluble sugars, especially the disaccharide sucrose, accumulated in a greater proportion at 2,580 m asl. The low sucrose content in San Francisco fruits (1,800 m asl) could have been caused by a higher temperature (+6.2°C) that accelerated metabolism and respiration more than in the fruits grown at 2,580 m asl.