INTRODUCTION
Climate variability and global warming strongly affect agricultural food production in particular regions as tropical and subtropic areas of the world. Brazil is one of the largest producers and exporters of poultry and livestock products globally, and the performance of this market may be affected by variations in the quality of soybean and corn crops, which are primary substrates used in animal feed (Bhardwaj et al., 2021). Increases in the quality and productivity of the corn crop due to geographical expansion and the diffusion of new technologies, combined with a good diet and inherent conditions linked to the species, enhances the development of the avian market (Venkataramana et al., 2014).
There are reports of corn crops produced at various times of the year with the increasing appearance of phytopathogenic microorganisms that provoke the production of mycotoxins (Shen et al., 2018; Bhardwaj et al., 2021). The primary toxigenic organisms belong to the genera Fusarium, Aspergillus, and Penicillium (Bhardwaj et al., 2021). Fusarium verticillioides produces fumonisin, a toxic secondary metabolite (Priyanka et al., 2015; Shen et al., 2018).
Fumonisin production depends on the expression of biosynthetic enzymes that depend on environmental and nutritional factors via several signaling pathways (Walker et al., 2016). Contamination with toxins FB1 and FB2 produced by F. verticillioides (Van Cleemput et al., 2019) occurs at various stages of corn production, causing rotting and seedling rust, posing risks for all sectors involved in food manufacture (Morales et al., 2019; Oldenburg et al., 2017). There are possible risks associated with the production of the corn crop at various stages of production; these involve rot and rust of seedlings that risk reducing the quality of the products and compromising food manufacture distribution channel. These contaminations also generate economic losses for agribusiness, attributable to the disposal of food or feed. There are also public health risks associated with poor performance and immunosuppression (Oldenburg et al., 2017; Morales et al., 2019).
Fumonisin have 28 analogs divided into four groups, identified as series A, B, C, and P (Bhardwaj et al., 2021; Shen et al., 2018). The B series (FB1, FB2 and FB3) contains the most relevant fumonisin and are the molecular forms most often produced by F. verticillioides with the most significant toxicity (Lerda, 2017). FB1 is widely distributed geographically and frequently occurs in corn (Lerda, 2017). Foods contaminated with FB1 can lead to diseases such as leukoencephalomalacia in horses (Van Cleemput et al., 2019), pulmonary edema in pigs (Haschek et al., 1992), nephrotoxicity in rodents, and esophageal cancer in humans (Van Cleemput et al., 2019). In poultry, the toxin causes variations in immunity, characterized by hepatic congestion (Murugesan et al., 2015), intestinal congestion (Bouhet & Oswald, 2007), necrotic enteritis, and coccidiosis (Grenier et al., 2016).
There are reports that in birds fed with the presence of fumonisin, there are changes in immunity due to immunosuppression, decreases in humoral immunity, and suppression of lymphocytes with hepatic congestion (Murugesan et al., 2015), intestinal congestion (Bouhet & Oswald, 2007), necrotic enteritis, and coccidiosis (Grenier et al., 2016). Murugesan et al. (2015) and Yarru et al. (2009) found that concentrations of 1.0mg/kg and 2.0mg/kg of AFB 1 caused decreases in hepatic gene expression of superoxide dismutase, glutathione S-transferase and with an increase in the relative gene expression Interleukin 6 and cytochrome p450 1A1 and 2H1 Yarru et al. (2009). In birds fed 2.0mg/kg of AFB 1, other liver genes were negatively regulated, including those associated with energy production and fatty acid metabolism (carnitine palmitoyltransferase), growth and development (insulin-like growth factor 1), antioxidant protection (glutathione S-transferase), detoxification (epoxide hydrolase), coagulation (coagulation factors IX and X), and immune protection (interleukins). By contrast, genes associated with cell proliferation (ornithine decarboxylase) were positively regulated Yarru et al. (2009).
The contamination of birds with FB1 negatively affects international trade and the economies of developed countries, giving rise to stricter supplier standards. Regulatory controls prevent the importation and local sale of products contaminated with fumonisin, directly affecting international trade in food products and feed (Alberts et al., 2016). In this context, it is essential to focus on preventing factors that can affect poultry performance and production. FB1 contamination adversely affects international trade and the economies of developed countries, giving rise to more stringent supplier standards. Regulatory controls prevent the importation and the local commercialization of products contaminated with fumonisin, directly affecting the international trade of food products and feed (Alberts et al., 2016).
It is essential to focus on the prevention of factors that may affect performance and poultry production. For this reason, it is essential to study the impact of FB1 on the poultry production chain. Therefore, our main objective was to identify a practical situation in which a corn crop from the 2015/2016 harvest could be proven to be infected by F. verticillioides to establish safe levels of this mycotoxin by evaluating crops in the 2015/2016 years using experimental culture from commercial hybrids. We also determined the zootechnical and histopathological parameters that would allow establishing minimum concentrations of FB1 that did not cause toxicological damage in birds of the COBB 500® lineage and contribute to the design of future studies involving the use of corn for human consumption.
MATERIALS AND METHODS
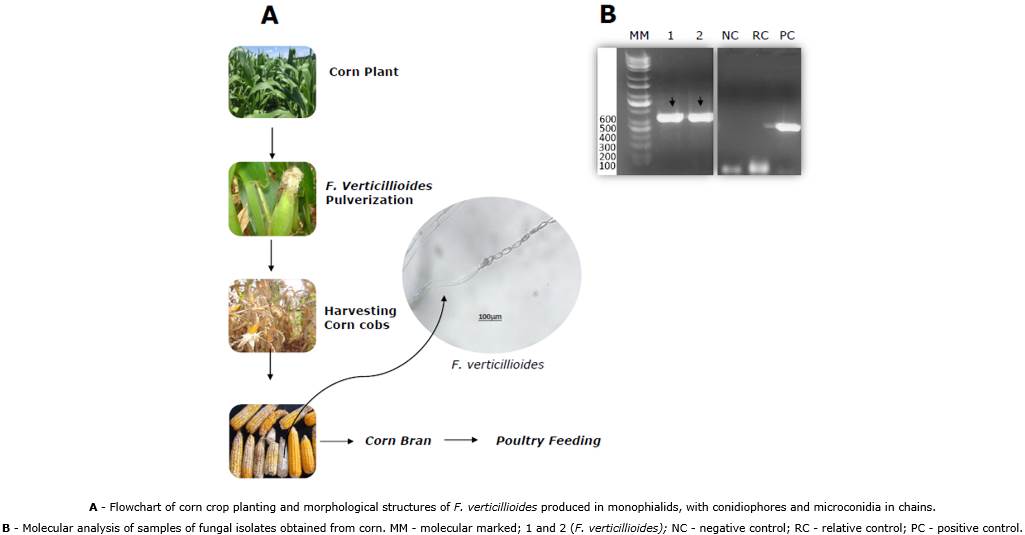
Identification, and susceptibility analysis of corn hybrids infected by Fusarium spp. This material was obtained from crops located in Gurupi city from Tocantins/Brazil (11º43'45"S/49º04'07"W). We created a mini-station to mimic a favorable microclimate at the Federal University of Tocantins, and we evaluated the selection of hybrids of commercial corn with greater susceptibility to Fusarium spp. Ten commercial hybrids were infected. For the isolation of the pathogen, symptomatic seeds were used and analyzed for the presence of F. verticillioides. Then, the fungus was transferred to Petri dishes containing BDA culture medium (200g potato, 20g dextrose, 20g agar, and 1000mL of distilled water). The isolates were identified based on their typical morphology, charaterized by the presence of long chains of microconidia produced in monofials. The image was taken under a Leica DM1000 LED optical microscope Leica DM1000 LED (Software Application Suite version 4.9.0) with 100x objectives, coupled with a microcomputer and digital camera Leica.
The monosporic culture technique was used, in which a suspension of conidia of the fungus was placed in 5mL of distilled and sterilized water in Petri dishes containing agar-agar culture medium (2%). After 24 hours of incubation at room temperature, germinated conidia were observed. The germinated conidia were isolated in experimental tubes and Petri dishes containing BDA culture medium. Koch's postulates were satisfied in healthy plants of the hybrid 32R48YH, at phenological stage R1, with inoculations of the fungus suspension in the style-stigmas with 5 x 105 conidia/mL. Samples were sent to the Agronomic Laboratory (Campinas, SP) for PCR analysis and genetic sequencing. DNA from F. verticillioides was extracted according to the protocol described by Doyle & Doyle (1990) using partial amplification of the specific calmodulin gene of 578bp. The primers were used Ver-1 (5'-CTT CCT GCG ATG TTT CTCC-3 ') and Ver-2 (5'-AAT TGG CCA TTG GTA TTA TAT ATCTA-3'). The purpose was to perform sequencing using specific primers to amplify and analyze the ITS1-5.8S regions of the calmodulin 578bp gene sequence that is a reference for identifying F. verticillioides (Rosa Junior et al., 2019).
PCR assay. The PCR reaction for the detection of F. verticillioides was carried out using a mixture of 5.0µL of the buffer (100mM Tris-HCl pH 8.5, 500mM KCl 1X), 1.0µL of dNTPs (0.2mM), 1.5µL of MgCL2, 2.5µL of sense and antisense primers (0.5mM), 1.0µL of Taq DNA polymerase (LGC) enzyme (5U/µL) and 5.0µL of total DNA. The final reaction volume was adjusted with Milli-Q water to 50.0µL. The thermocycler regime for this reaction was 95ᵒC for 5 minutes, 25 cycles of 1.5 minutes at 95ᵒC, 1.5 minutes at 62ᵒC, 1.5 minutes at 72ᵒC, and a final extension step at 72ᵒC for 10 minutes. This amplification product was subjected to conventional electrophoresis on a 1% agarose gel and visualized on a UV transilluminator.
Feed formulation. Ears with contaminated corn kernels were harvested in the phenological phase R6 (physiological maturity 98) and placed in automatic dryers, crushed, and ground for feeding birds in the T2 group (those give contaminated feed). A T1 control group was established consisting of birds fed with contamination-free feed. COBB 500® birds were used to evaluate the effect of F. verticillioides FB1 on zootechnical performance and histopathological damage. There was no addition of antibiotics or chemotherapeutic agents, or products of animal origin in any feed. The birds were monitored for 21 days to assess zootechnical performance and histopathological damage.
Analysis of fumonisin in corn hybrids. Samples of contaminated feed and control were sent to the Laboratory of Analytical Mycotoxicology (LAMIC) of the Federal University of Santa Maria (Santa Maria, RS). Ten grams of sample were crushed through 2.0-mm meshes and extracted with 50-mL water/acetonitrile (1:1, v/v) for 5 min in a high-speed blender. The extracts were then filtered; 20mL aliquots were diluted in acetonitrile/water with formic acid 1% (1:1, v/v) and subsequently analyzed using liquid chromatography-mass spectrometry (LC-MS/MS), according to Dall'Asta et al. (2008), to determine the presence, concentration, and type of fumonisin FB1 and FB2. These values were calculated based on the calibration curve at six concentration levels ranging from 125 to 10,000mg/kg of FB1 and FB2 purified standard (Sigma Aldrich).
Experimental design in COBB 500 birds. The experiments design in COBB 500® birds were conducted in the experimental aviary of Veterinary Medicine and Animal Science at the Federal University of Tocantins, at the campus of Araguaína city, Tocantins/Brazil (07°11’28”S / 48°12’26”W). We used chicks of the COBB 500® strain on their first day of life. The mean weight was 38.7g. We randomly distributed the chicks in experimental cages to consume feed until the 21st day of life. They were distributed in a completely randomized experimental design with two treatments, T1 (feed without fumonisin) and T2 (corn contaminated with micotoxins from fungi at 2.78μg/g). The experimental group included 160 animals, divided into two groups: T1 without treatment, that is, the control group with no fumonisin in the diet; and the T2 group in which corn was contaminated with 2.78μg/g of fumonisin. The experimental group was distributed in a completely randomized design with two T1 and T2 treatments with ten replicates and eight birds in each group. On the 1st day of age, the birds were weighed and randomly distributed in the cages of each experimental group, receiving diets without fumonisin (Group T1) and diets with fumonisin at 2.78μg/g (Group T2). The experiment was conducted for up to 21 days. Zootechnical performance parameters were obtained from the 1st to the 21st day of life. Feed intake (FI), weight gain (WG), and feed conversion (FC) were calculated. WG was calculated as the difference between the final weight and the initial weight. Feed consumption was calculated as the difference between total feed supplied and leftovers obtained. The FC was calculated as the ratio between the total FI and the WG, corrected for the weight of the dead birds. On day 21, birds and feeds were weighed for determination of FI, WG, and FC.
Histopathological analysis. Two birds of each replicate (totaling 20 birds of each treatment, T1 and T2) fasted for 12 hours and intramuscularly administered ketamine (40mg/kg) with xylazine (2mg/kg). They were sacrificed by cervical dislocation and were subsequently bled and eviscerated for visual and histological assessment of the internal organs (liver, small intestine, and heart) (Lerda, 2017). The experiment was conducted based on the norms of the experimental use of animals approved by CEU/UNILA under protocol number 001/2018.
For macroscopic analysis of the viscera, a necropsy was performed on 40 birds (20 birds in the T1 group and 20 birds in the T2 group). After evaluating the viscera, fragments of 3-mm thickness were removed, and these were placed in containers containing 10% formaldehyde buffered for at least 24 hours. The liver, heart, and small intestine were removed at necropsy and fixed in 10% buffered formaldehyde for histopathological analysis. After this procedure, the fragments were dehydrated in ethyl alcohol in increasing concentrations, diaphanized by xylol, infiltrated, and set in paraffin. The blocks were cut in a microtome set to 5µm.
The sections were subjected to hematoxylin-eosin staining, then a descriptive analysis of the results was performed. The liver, heart, and intestine were evaluated based on the histopathology observed after the end of the experiment. Intestine, liver, and heart fragments were collected, cleaved, and fixed in 10% buffered formaldehyde. After this procedure, the fragments were dehydrated in ethyl alcohol in increasing concentrations, diaphanized by xylol, impregnated, and included in paraffin. The blocks were cut in a microtome adjusted to 5 µm. The sections were subjected to hematoxylin-eosin staining. Then, a descriptive analysis of the results was performed. Slides were made for the hematoxylin-eosin staining and microscopy (400x).
Analyses were performed to determine the presence and degree of alterations in the T1 and T2 groups for vacuolations, villous atrophy, goblet cell hyperplasia, and hemorrhage or hepatic necrosis. Scores were as follows: 0) absence; 1) scant, up to 25% of the field; 2) moderate, greater than 25% but less than 50% of the field of observation; and 3) severe, greater than 50% of the field of observation (Cangussu et al., 2018; Rashidi et al., 2020).
Statistical analysis. The data were subjected to one-way Analysis of Variance and F-test with 95% confidence interval to evaluate the zootechnical parameters and histopathological data using software Assistat 7.7.
RESULTS AND DISCUSSION
Corn grains infected with fumonisin from F. verticillioides were evaluated in the feed. These results suggest to understanding the effect on animal performance evaluated by zootechnical and histopathological parameters. The morphology of isolates obtained from ears of dry corn kernels in the stage (R6) was evaluated. Data showed conidiophores with microconidia in long chains of microconidia in monophyllides, characteristic structures of F. verticillioides (Figure 1). To confirm the molecular profile, we used amplified ITS1-5.8S regions referring to the 578bp calmodulin gene sequence used to identify F. verticillioides (Figure 1b). The genes were sequenced, revealing 99% and 99.9% sequence similarity (Query cover) for F. verticillioides (Blast, NCBI, Icl/Query_25443, Icl/Query_10149), and plant health report (3162/2017-AGR). In the field experiment, the hybrid P32R48YH was selected for presenting the characteristics of susceptibility to disease and with the spraying of conidia of F. verticillioides during stage R1. The ears in stage R6 showed the morphological characteristics of infections with F. verticillioides, with levels of 2.78μg g-1 of fumonisin FB1 being determined in the feeding of birds.
Poultry fed control feed (T1) and feed contaminated with F. verticillioides and containing 2.78μg/g of fumonisin FB1 (T2). We performed the subsequent experiments with 160 COBB 500® birds, 80 birds in each group (T1 and T2) and were evaluated for animal performance monitored by zootechnical and histopathological parameters of sections of liver, heart, and small intestine.
The weight data from the birds were obtained at the beginning and the end of each week as follows: FI/bird of 1136.10g, FC/bird of 1.53g, and final WG of 746.14 g after 21 days consuming control feed (T1) (Table 1). Data on birds treated with F. verticillioides-contaminated corn (T2) were: FI/bird of 1110.38g, FC/bird of 1.50 g, and final WG of 743.63 g (Table 1). We also evaluated the individualized behavior of COBB 500® birds over 3 weeks, and WG was used as one of the performance parameters of birds fed with contaminated feed (T2) with F. verticillioides fumonisin FB1 and control feed (T2) without the presence of FB1 fumonisin. We did not find significant variations between the treatment groups T1 and T2 (Table 1), with an F-value > P-value.
The histopathological study was carried out using 20 birds in each group (T1 and T2). We evaluated histopathological data of the liver, heart, and small intestine of birds to investigate the occurrence of histopathological lesions such as vacuolations, villous atrophy, goblet cell hyperplasia, bleeding, and hepatic necrosis (Figure 2, Table 2). However, histological findings revealed slight changes with vacuolizations of the same intensity and distribution in both groups in sections of livers and hearts (Figure 2). This result suggests that the concentrations of 2.78 μg/g of FB1 obtained from the hybrid corn P32R48YH infected with F. verticillioides did not cause significant changes, either in terms of variation in zootechnical parameters or histopathological parameters.
Table 1 Zootechnical parameters of poultry consuming Fumonisin of corn contaminated.
| Treatment | FI (g/birds) | FCR (g/day) | WG (g) | FE (%) |
| T1 | 1136,10ª (18,35) | 1,53ª (0,03) | 805,02ª (14,12) | 70,85ª |
| T2 | 1110,38ª (19,01) | 1,50ª (0,02) | 792,13ª (13,50) | 71,33a |
Mean values followed by the same letter were not significantly different at the level of 5% probability; FI - Feed Intake; FCR - Feed Conversion; WG - Weight Gain; FE - Feed Efficiency.
Table 2 Score of histopathological data from sections of liver, small intestine, and heart of COOB 500® birds fed with ration contaminated with Fumonisin FB1 (T2) and birds fed with control ration without contamination (T1).
| Treatment | Chickens’ organs | Score | P |
| T1 | Liver Heart Small intestine | 0-1 0-1 0-1 | ns ns ns |
| T2 | Liver Heart Small intestine | 0-1 0-1 0-1 | ns ns ns |
The scores were as follows: 0) absence; 1) scarce, up to 25% of the field; 2) moderate, greater than 25%, but less than 50% of the observation field; and 3) severe, greater than 50% of the observation field.
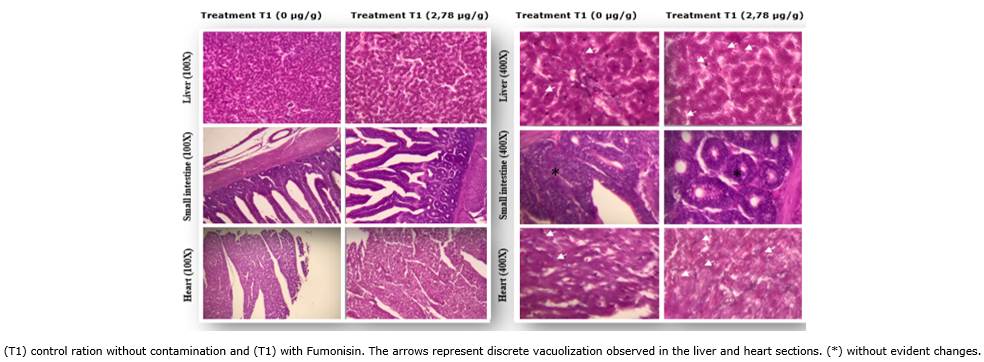
Figure 2 Histopathological data from sections of COOB 500® birds fed with ration contaminated with Fumonisin.
The existence of mycotoxins in agricultural products has become a global concern. For these reasons, the threat of mycotoxins poses an even greater concern for human and animal food security (Knutsen et al., 2018). Mycotoxins such as aflatoxins, ochratoxins, trichothecenes, zearalenones, and fumonisin are economically important because of their associated toxicities, including nephrotoxicity, neurotoxicity, carcinogenic properties, and immunosuppressive effects, especially when present in high amounts (Shen et al., 2018). In small amounts, mycotoxins can reduce appetite and WG in animals and cause diarrhea in humans (Kovalsky et al., 2016). The International Agency for Research on Cancer (IARC) classified fumonisin (FB1 and FB2) in the 2B carcinogen risk group concerning oesophageal cancer in humans (Shen et al., 2018).
Mycotoxins affect both public health and have economic implications for agricultural production and the availability of food worldwide. Approximately 25% of all agricultural products are contaminated by mycotoxins (Bhardwaj et al., 2021). For these reasons, the quality of products intended for food must be assessed before use to avoid any danger to the health of humans, animals, or the environment. Several maximum permissible concentrations have been proposed for various mycotoxins in different types of food. These restrictions vary from country to country due to differences in the origin of food, the possibility of contamination and ingestion at meals; there is currently no international legislation regulating permissible levels (Kovalsky et al., 2016; Smith et al., 2016).
The European Union (EU) sets a maximum permissible level of 200µg/kg for FB1 + FB2 in processed corn-based foods. The European Food Safety Authority (EFSA) established the tolerable daily intake of 1µg/kg of body weight per day for humans (Knutsen et al., 2018). The maximum tolerated level for birds, in general, is 0.02μg/g Comissão Europeia, 2006. According to the US Food and Drug Administration, the maximum permissible levels of FB1 + FB2 for laying hens are 0.03μg/g and 0.1μg/g for broilers (Van Egmond & Jonker, 2005). Brazilian legislation also aims at control and quality concerning mycotoxin contamination and consequently ensures the population's health (Ministério da Saúde do Brasil, 2011). The concentrations of subclasses FB1 + FB2 are permitted up to 1μg/g in products of animal origin (Ministério da Saúde do Brasil, 2011). Ministério da Saúde do Brasil, 2011). Analytical methods such as high-performance liquid chromatography coupled with sequential mass spectrometry are used to identify and quantify mycotoxins, thereby helping to guarantee compliance with the legislation.
Our data revealed that the same in the concentration of 2.78μg/g fumonisin FB1 obtained in the P32R48YH hybrid that has the highest susceptibility for F. verticillioides infection not found significant histopathological damage or alteration in the zootechnical parameters in COBB 500® birds fed with feed containing this concentration of FB1 over 21 days. Wyatt & Henry (1993), also studied concentrations 0, 0.2, 0.4, and 0.8μg/g of FB1 purified in birds up to 21 days and did not observe significant variations in WG or FC. At concentrations of 1 to 2μg/g of FB1, some reports indicated a low influence on animal performance. FB1 levels greater than 3μg/g caused diarrhea, decreased food intake, and weight loss with swelling of the liver, kidneys, and hepatic necrosis (Ledoux et al., 1996).
The toxic effects of fumonisin are directly related to the dose ingested by the animal (Ledoux et al., 1996; Murugesan et al., 2015). However, Zachariasova et al. (2014) stated that it is necessary to observe the concentration and the contact time of FB1 in birds because both determine the appearance of lesions that start in the mouth and evolve to damage to the intestine and liver. The primary lesions by FB1 have been reported in bird livers, with FB1 levels greater than 3μg/g (Wielogorska et al., 2016). The lesions begin after swelling with erosions in the stomach and intestine, culminating in hemorrhage and death (Kovalsky et al., 2016). Villous atrophy and goblet cell hyperplasia were also observed in the jejunum (Lerda, 2017). Rauber et al. (2012) reported the appearance of liver lesions such as hepatocellular vacuolization, bile duct hyperplasia, hepatocellular degeneration, bile duct proliferation, and lymphoid hyperplasia in birds fed high concentrations of FB1 (100 and 200μg/g) without heart or small intestine lesions. Our histopathological data revealed only mild changes of vacuolations of the same intensity and distribution in livers and hearts in both treatments (T1 and T2), suggesting that 2.78μg/g fumonisin obtained from the hybrid corn P32R48YH infected with F. verticillioides did not cause significant changes in histopathology, even though the dose was approximately three times above the maximum tolerance limit determined by Anvisa/Brazil.
CONCLUSIONS
Study presents a relevant scientific contribution with the P32R48YH hybrid corn at stage R1 being the more susceptible to F. verticillioides infection with a maximum yield of fumonisin. In addition, the fumonisin produced of P32R48YH hybrid corn no caused impact on the health of poultry. This data will assist in establishing minimum and safe concentrations of FB1 and improve the criteria for poultry meat production.