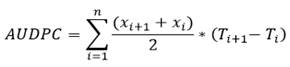INTRODUCTION
The goldenberry, or also known as Goldenberry or Uchuva (Physalis peruviana L.), is an Andean fruit species that has become an alternative for the economy of many countries as it stands out as an export product; its importance derives from the nutritional characteristics and medicinal properties (Chavez et al., 2019). In Colombia, cape gooseberry is the second most exported product after bananas; its cultivation offers great advantages because, being Colombia a tropical country, the permanent production of the fruit can be guaranteed for international markets (Ruiz et al., 2018; García et al., 2021). In Colombia, the producing departments are Boyacá, Cundinamarca, Antioquia, Nariño, Norte de Santander, Santander, Huila, Tolima, Cauca and Valle del Cauca, with Boyacá being the largest producer with 30.6% of the planted area, followed by Cundinamarca with 28.6% and Nariño with 13.4% (EVA, 2021).
Among the most important diseases of the crop, we can find vascular wilt (Fusarium oxysporum f. sp. phsali), dieback (Phoma physalidis), gray leaf spot (Cercospora physalidis), fruit spot (Alternaria sp.), and Gray mold (Botrytis cinerea); other diseases reported in Colombia, but of lower incidence are cottony rot (Sclerotinia sclerotiorum), stem rot (Pythium sp.), Anthracnose (Colletotrichum gloeosporioides), the bacteria Xanthomonas sp., Ralstonia solanacearum, nematodes such as Meloidogyne sp., Pratylenchus sp., and viruses such as leaf-roll virus (PLRV), Potato virus Y (PVY) and Andean mottle virus (APMV) (Ruiz et al., 2018; Diaz et al., 2019).
Vascular wilt caused by Fusarium oxysporum f. sp. physalis (FoPh) is one of the most limiting diseases for Goldenberry as it has generated losses between 80 and 90% (García et al., 2021; Simbaqueba et al., 2021). In the last decade, the department of Cundinamarca suffered at great lost of the crop and it was necessary to relocate it to Boyacá department; that is why the production of this fruit is concentrated in this place (Valderrama, 2018; Simbaqueba et al., 2018; Chávez et al., 2019; Simbaqueba et al., 2021). This fungus is difficult to manage due to soil contamination with the pathogen, something that occurs when harvest residues and affected plant tissues are not properly discarded, or when contaminated soil is moved (Valderrama, 2018). Plants can become infected with the fungal reproductive structures such as mycelium, conidia, and chlamydospores (resistance structures that manage to remain up to thirty years in soils), which germinate upon contact with host plant root exudates (Vásquez & Castaño, 2017; Joshi, 2018; Giraldo et al., 2020). Plants affected by FoPh are initially characterized by leaf chlorosis followed by generalized yellowing. Symptoms that are mixed with loss of turgor in branches and stems. In general, these symptoms tend to be unilateral, affecting one or two of the main stems; it is also common to see that they are bent leaving the fruits attached to them.
The progression of the disease ends up affecting the entire plant which eventually dies (Gordon, 2017; Gonzales, 2019; Chávez et al., 2019; Giraldo et al., 2020) When longitudinal cuts are made in the stems and branches, a light brown coloration of the parenchyma is observed, which in advanced stages turns brown; comparatively, the bark tissues are seemingly healthy (Valderrama, 2018; Agudelo, 2020).
Disease management is based on preventive measures that include not planting in plots with a history of incidence by F. oxysporum, the use of pathogen-free propagation material, avoiding unnecessary wounds during cultivation, weed control to reduce excess moisture, avoiding soil waterlogging, eradication of diseased plants, crop rotation, and solarization (Moreno et al., 2019; Berruezo, 2018; Chávez et al., 2020). Non-preventive alternatives include biological control and the search for resistant cultivars; the latter is seen as one of the most effective and economically profitable measures for disease management in the field (Vásquez & Castaño, 2017; Rodríguez & Pedraza, 2019).
Regarding the resistance response to Foph to avoid losses in the producing areas, numerous efforts have been made, such as the one done by Pulido et al. (2011), who carried out bioassays with 70 goldenberry accessions and through cluster analysis, concluded that three of the evaluated materials presented resistance to the pathogen. Rodríguez (2013), on the other hand, through pathogenicity tests in the field, identified two introductions of Physalis with significant values of resistance to vascular wilt. Osorio et al. (2017) identified promising accessions with different degrees of resistance, as well as 16 markers associated with the resistance response. Mayorga et al. (2019) found genetic materials with desirable agronomic traits and excellent response to Foph attack and considered them important for breeding schemes.
Until 2018, the department of Nariño did not present a historical record of incidence of vascular wilt ( Agencia UNAL, 2018). However, foci of the disease have been identified, so the efforts of producers and technicians should be oriented to avoid its appearance and dissemination, due to the considerable losses in yields that this fungus causes.
The objective of this work was to evaluate the reaction of Goldenberry genotypes against Fusarium oxysporum f. sp. physali (FoPh) under greenhouse conditions, with the purpose of identifying sources of resistance that could be used in breeding programs for the production of improved cultivars with resistance to the disease.
MATERIALS AND METHODS
Location. The evaluation of the 40-goldenberry genotypes and their reaction to Fusarium oxysporum attack was carried out in the greenhouse located in the Agrosavia C.I. Obonuco facilities, at 2760masl and with an average temperature of 13°C.
Genetic materials. Forty genetic materials were used; 9 double haploid lines and 11 genotypes of the Fusarium group belong to the Colombian Agricultural Research Corporation AGROSAVIA Tibaitatá, 19 genotypes from the University of Nariño and a commercial control, which is a selection made by cape gooseberry farmers in the department of Nariño (Table 1). The seeds were initially sown in germination trays with peat substrate. When the seedlings presented 3 to 4 true leaves, they were taken to 1kg bags containing sterile soil and irrigated four days a week for 4 hours by mist irrigation. Fertilization was edaphic.
Table 1 Genetic materials evaluated for their reaction against to Fusarium oxysporum f.sp. physali.
| AGROSAVIA | UDENAR | |||
|---|---|---|---|---|
| 12U347 | 09U086 | UN01 | NEIRA | |
| 12U350 | 09U089 | UN03 | UN45 | |
| 12U352 | 09U099 | UN13 | UN49 | |
| 12U357 | 09U116 | UN14 | UN52 | |
| 12U360 | 09U128 | UN19 | SILVANIA | |
| 12U368 | 09U136 | UN26 | PERU | |
| 12U374 | 09U138 | UN30 | COLOMBIA | |
| 12U377 | 09U140 | UN34 | PURACÉ | |
| 12U399 | 13U407 | UN35 | KENIA | |
| ANDINA | 13U408 | UN36 | CONTROL | |
During transplantation and growth, a mixture of DAP+ Agriminis® (0.5g/plant) was applied, during the flowering stage a mixture of calcium nitrate+10-30-10 (2g/plant) was used and during production, potassium nitrate (3g/plant). Monthly applications of 15-15-15 (5g/plant) were then made, which were increased over time. A V-shaped trellis system was used; with pruning every 20 days, weed control was manual and harvests were scheduled twice a month. To control the stem borer, Exalt® was applied at the dose suggested by the manufacturer (2mL/L).
Isolation and purification of the pathogen. The pathogen strain used in this study was obtained from one of the experimental plots located in Puerres. For its isolation, samples were taken from plant stems showing vascular necrosis; diseased tissue cuts of approximately 3 mm were made, and each cut underwent the disinfection protocol (1% sodium hypochlorite, 70% alcohol, and sterile distilled water washings). The disinfected tissues were seeded in PDA culture medium (39g/L of water) and incubated for eight days at 22°C. Colonies that presented a whitish cottony appearance and pink color on the underside were replicated in the same medium for isolation and subsequent identification. A small amount of mycelium was then extracted with the aid of a dissecting needle and observed under the microscope at 40X, identifying microconidia with 0 to 3 septate macroconidia characteristic of Fusarium oxysporum (Carmona et al., 2020). The isolation used was molecularly confirmed by DNA sequencing of the ITS and EF1α genes carried out in the molecular biology laboratories of Agrosavia at C.I. Tibaitatá.
Inoculation by root immersion. The inoculum was obtained in PDA culture medium; the Petri dishes seeded with the pathogen were incubated for eight days. The conidia were removed with the help of a bacteriological rake by adding 200mL of sterile distilled water with 0.1% Tween 80, then filtered through a gauze. The suspension was adjusted to 1x106conidia/mL calibrated using a hematocytometer, following the methodology described by Arellano (2018) and Agudelo (2020). When the goldenberry plants were 20 cm high, they were removed from their plastic bags; the roots were washed with sterile distilled water and with the help of scissors disinfested with 1%, quaternary ammonium. The apexes of the main root were cut; then they were submerged from the stalk base to the root for 30 minutes in 250 mL plastic cups containing the conidial suspension. Finally, the plants were planted in bags with sterilized soil. Arellano (2018), Ángel et al. (2018) and Agudelo (2020) have used this method.
Traits evaluated
Plant height (cm). The height of each genotype and its replicates were recorded weekly by using a tape measure.
Severity (%). Severity (Table 2) was recorded every eight days, using the scale proposed by Garcés et al. (2017).
Table 2 Scale used to qualify the degree of severity of vascular wilt caused by Fusarium oxysporum.
| Severity | Percentage | Traits |
|---|---|---|
| 0 | 0 | No symptoms manifested |
| 1 | 10 | Mild chlorosis, without necrotic lesions or more than 10% of the total foliage is wilted and/or chlorotic. |
| 3 | 25 | Leaves wilted and/or chlorotic |
| 5 | 50 | 50% of the leaves show the beginnings of wilting |
| 7 | 75 | 75% of foliage severely wilted |
| 9 | 100 | Dead or severely infected plants showing practically all their foliage wilted, with chlorosis, necrosis, and/or premature defoliation. |
Area under the disease progress curve (AUDPC). To compare the differences between genetic materials from disease severity values, the AUDPC calculation was performed, this parameter incorporated the speed of disease progression and severity into a single value. That is, accumulation of daily values of the percentage of infection interpreted directly without performing any transformation (Chañag et al., 2017; Sánchez et al., 2017; Bocianowski et al., 2020), Equation 1.
Where: Xi =Proportion of affected tissue under observation I, Ti+1-Ti = time in days between two readings, n =Total number of observations.
Incidence (%). The number of diseased plants was evaluated weekly- The incidence was obtained by applying the formula:
Incidence (%) = (Number of sick individuals/ total individuals) x 100
Vascular discoloration. Once the observations were finished, the plants were removed from the plastic bags and a transversal cut was made; the upper, middle, and lower part of each genotype was evaluated, using the scale (Table 3) proposed by Garcés et al. (2017).
Susceptibility index. The mean and standard deviation statistics were used; then a relative value was assigned to the evaluated traits to obtain a score that allowed a classification of the tested genotypes.
Experimental design and statistical analysis. The Completely Randomized Design was used with 39 genotypes, a commercial control and five plants like replications per experimental unit, of which one was a control inoculated with water. During the entire experiment, 28 evaluations were made; it should be noted that after reading 14, the surviving genotypes were inoculated again to verify that the resistance presented was not associated with escape. Analysis of Variance (ANOVA) and Tukey's mean comparison tests at 95% probability were carried out using the statistical program Statgraphics Centurion XVII.
RESULTS AND DISCUSSION
Plant height. The ANOVA for plant height indicated significant statistical differences among the genetic materials evaluated (P<0.05). The Tukey test for comparison of means showed that the genotype that presented the greatest growth was 12U399 with 49.94cm, followed by 09U138 with 46.73cm; results that did not differ statistically from the commercial control (35.09cm), but from UN35 that showed the lowest average (9.61cm); 13U407 and UN34 showed heights of 44.73, 44.21, 43.92 and 41.35cm respectively, with no statistical difference with each other; the other plants showed averages fluctuating between 40.91 and 22.64cm.
On the other, the growth for 09U136, 12U374, Puracé, and UN49 was 18.67, 18.22, 17.15, and 14.32cm. In that regard, Insuasty et al. (2014) obtained low height averages by studying the pathosystem pea-Fusarium oxysporum, and they concluded that growth was reduced due to the pathogen's ability to colonize roots, which in turn prevents adequate nutrition; Wang & Jeffers (2002) state that hostas (Hosta spp.) plants, when inoculated with strains of Fusarium ostae experienced growth retardation, Rose et al. (2003) reported that squash (Cucurbita pepo) plants infected with Fusarium oxysporum f.sp radiciscucumerinum showed a lower height compared to the commercial control, thus showing the negative effect of the pathogen on this trait.
Alvarado (2005), when evaluating pathogenic strains of Fusarium sp. in colored calla lilies (Zantedeschia spp.) indicates that one of the most important aspects affecting this fungus is size; at the end of the experiment, the calla lilies, besides being dwarf, had small and thin leaves, showing poor root development. F. oxysporum is a fungus that invades the cortical cells of the root intercellularly during the infection process; it enters the vascular system through the xylem and begins to produce microconidia, which rapidly colonize the plant generating obstructions by hyphae, which prevent communication between the cells of the conducting vessels, blocking the flow of water and nutrients and directly interfering with plant growth (Marín et al., 2018; Chávez et al., 2019; Srinivas et al., 2019; Giraldo et al., 2020).
The results obtained for this trait (Table 4) in genotypes 12U399 (49.94cm) and 09U138 (46.73cm) indicate resistance to FoPh. In this regard, Rodríguez (2020) states that in resistant crops, when the pathogen attack is detected, flavonoids such as catechins and their oxidation products inactivate the enzymes, so that colonization is confined to the initial infection points. Bani et al. (2018) point out that resistant plants are able to prevent the advance of the fungus by sealing the xylem by means of gels or gums constituted by polysaccharides of high molecular weight.
Area under the disease progression curve (AUDPC). From the severity data, the area under the disease progress curve (AUDPC) was obtained; value that in turn was calculated using the trapezoidal integration model (Alves et al., 2017; Castellanos et al., 2021), finding significant statistical differences (P<0.05) in disease intensity over time for the genotypes evaluated. Tukey's mean comparison test indicated that genotypes 09U138 and 12U399 differed from the others, even from the commercial control (975 units) by presenting the lowest AUDPC values with zero units (Table 4), followed by Peru with 529 units. UN34, 13U407, and 09U089 revealed AUDPC of 803, 805, and 815 respectively without statistical differences with each other; the rest of the genotypes showed AUDPC ranging from 828 to 1340 units.
The highest values (Table 4) were for 09U128, 09U086, 09U136, Puracé, UN49, and UN35 with 1398, 1400, 1403, 1428, 1473, and 1519 units, which were not different from the commercial control (975 units). Materials 09U138 and 12U399 were the least affected by the disease and apparently could be classified as resistant to F. oxysporum f. sp. physali attack. It is worth mentioning that at the end of evaluation 14, a second inoculation of the surviving genotypes was performed by the root immersion method, as explained in the methodology, to verify that the absence of symptoms was not associated with escape. The results validate the report by Osorio et al. (2017), who through conglomerate analysis identified the resistance of different Goldenberry accessions to FoPh attack, the genotype 09U138 was characterized by low severity and lower AUDPC.
Table 4 Tukey's means test for plant height, severity and incidence of the Goldenberry genotypes evaluated.
| GENOTYPES | HEIGHT | GENOTYPES | AUDPC | GENOTYPES | INCIDENCE | |||||
| 12U399 | 49.94 | A | 12U399 | 0 | A | 12U399 | 0 | A | ||
| 09U138 | 46.73 | AB | 09U138 | 0 | A | 09U138 | 0 | A | ||
| 12U357 | 44.73 | ABC | PERÚ | 529 | AB | PERÚ | 25.00 | B | ||
| NEIRA | 44.21 | ABC | UN34 | 803 | ABC | 09U089 | 50.00 | C | ||
| 13U407 | 43.92 | ABC | 13U407 | 805 | ABC | UN34 | 75.00 | D | ||
| UN34 | 41.35 | ABC | 09U089 | 815 | ABC | 12U352 | 75.00 | D | ||
| 12U377 | 40.91 | ABCD | SILVANIA | 828 | BC | UN14 | 75.00 | D | ||
| SILVANIA | 40.56 | ABCDE | UN26 | 880 | BC | 12U360 | 75.00 | D | ||
| KENIA | 38.82 | ABCDEF | UN52 | 936 | BC | 09U099 | 75.00 | D | ||
| UN52 | 38.47 | ABCDEF | CONTROL | 975 | BC | SILVANIA | 75.00 | D | ||
| 09U089 | 37.62 | ABCDEF | 12U352 | 1031 | BC | 13U407 | 75.00 | D | ||
| 12U352 | 37.15 | ABCDEF | UN36 | 1053 | BC | CONTROL | 75.00 | D | ||
| 12U350 | 37.01 | ABCDEF | NEIRA | 1071 | BC | UN52 | 75.00 | D | ||
| UN36 | 36.66 | ABCDEFG | 12U368 | 1085 | BC | UN26 | 81.25 | E | ||
| UN45 | 36.36 | ABCDEFG | UN14 | 1090 | BC | UN01 | 100.00 | F | ||
| UN13 | 36.32 | ABCDEFG | ANDINA | 1099 | BC | UN36 | 100.00 | F | ||
| UN26 | 35.78 | ABCDEFG | UN13 | 1123 | BC | UN45 | 100.00 | F | ||
| PERÚ | 35.75 | ABCDEFG | COLOMBIA | 1134 | BC | PURACE | 100.00 | F | ||
| CONTROL | 35.09 | ABCDEFG | 09U099 | 1148 | BC | UN49 | 100.00 | F | ||
| 12U360 | 33.45 | ABCDEFG | UN45 | 1151 | BC | UN19 | 100.00 | F | ||
| 12U347 | 33.10 | ABCDEFG | UN30 | 1155 | BC | UN13 | 100.00 | F | ||
| UN03 | 33.08 | ABCDEFG | UN19 | 1157 | BC | UN30 | 100.00 | F | ||
| UN19 | 32.86 | ABCDEFG | 13U408 | 1172 | BC | UN35 | 100.00 | F | ||
| 09U099 | 31.09 | ABCDEFGH | KENIA | 1179 | BC | UN03 | 100.00 | F | ||
| 12U368 | 29.75 | ABCDEFGH | 12U360 | 1248 | BC | 09U140 | 100.00 | F | ||
| UN01 | 29.70 | ABCDEFGH | UN01 | 1256 | BC | 12U347 | 100.00 | F | ||
| UN30 | 29.47 | ABCDEFGH | 12U347 | 1291 | BC | 12U350 | 100.00 | F | ||
| 09U128 | 29.32 | ABCDEFGH | 09U116 | 1305 | BC | 12U357 | 100.00 | F | ||
| COLOMBIA | 29.24 | ABCDEFGH | 12U377 | 1307 | BC | 09U086 | 100.00 | F | ||
| 13U408 | 29.05 | ABCDEFGH | 09U140 | 1307 | BC | 09U128 | 100.00 | F | ||
| UN14 | 26.29 | BCDEFGH | 12U374 | 1311 | BC | 09U136 | 100.00 | F | ||
| 09U086 | 25.22 | BCDEFGH | UN03 | 1318 | BC | 09U116 | 100.00 | F | ||
| 09U140 | 23.54 | CDEFGH | 12U350 | 1333 | BC | ANDINA | 100.00 | F | ||
| 09U116 | 23.18 | CDEFGH | 12U357 | 1340 | BC | COLOMBIA | 100.00 | F | ||
| ANDINA | 22.64 | CDEFGH | 09U128 | 1398 | C | KENIA | 100.00 | F | ||
| 09U136 | 18.67 | DEFGH | 09U086 | 1400 | C | NERIA | 100.00 | F | ||
| 12U374 | 18.22 | EFGH | 09U136 | 1403 | C | 12U368 | 100.00 | F | ||
| PURACE | 17.15 | FGH | PURACE | 1428 | C | 12U374 | 100.00 | F | ||
| UN49 | 14.32 | GH | UN49 | 1473 | C | 12U377 | 100.00 | F | ||
| UN35 | 9.61 | H | UN35 | 1519 | C | 13U408 | 100.00 | F | ||
Means with different letters indicate significant difference according to Tukey test (P≤0.05).
Regarding resistance, Pulido (2010) and Gayosso et al. (2021) explain that once pathogens overcome mechanical barriers to infection, plant receptors initiate signaling pathways that drive the expression of defense response genes that depend on their ability to recognize harmful molecules, carry out signal transduction, and respond defensively to a potential invader. Burbano (2020) and Kaur et al. (2021) additionally state that when a resistance gene identifies an avirulence gene in the pathogen, a process of cell death is activated in the infected cell, completely stopping colonization; thus, granting resistance to the plant, which is what possibly occurred with genotypes 09U138 and 12U399. However, it is recommended that these genetic materials be evaluated against other strains of the fungus. Pouralibaba et al. (2017), Bani (2018), and Joshi (2018) explain that Fusarium oxysporum colonization is restricted in cultivars resistant to the region of initial entry of the pathogen, due to occlusion of the vessels by gels, callose, and tyloses deposition.
The high AUDPC values observed in the rest of the genotypes apparently, indicated low amounts of callose and tyloses that were degraded by the pectolytic enzymes of the pathogen, which interfered in the activation of the defense system against Fusarium oxysporum, showing a high susceptibility and a compatible interaction where the pathogen initiates the infection in which it can multiply and progress systemically, invading other tissues triggering the disease (Chañag et al., 2017; Castro et al., 2020; Islas, 2021; Kaur et al., 2021).
For infection to be successfully achieved, the pathogen-plant interaction responds to a process where different sets of genes, must be mobilized that allow early host signaling, adhesion to the host surface, enzymatic breakdown of physical barriers, defense against plant antifungal compounds, and inactivation and subsequent death of host cells by secreted mycotoxins (Agrios, 2005).
The expression of symptoms in susceptible genotypes was observed after the second week of inoculation with the fungus, results that coincide with those reported by Pulido (2010) and Osorio et al. (2017), who affirms that in the case of Goldenberry the age of the plant does not influence the speed of infection and symptoms begin to be evident within the first two weeks after inoculation.
Figure 1 shows the initial and final severity of each of the genotypes evaluated, it can be clearly seen that almost all reached a final affectation of 100%, except for 13U407, UN26, Silvania, commercial control, UN14, UN34, and 12U352, whose severities ranged between 74±24.7% and 78±24.7%; on the other hand, Peru, UN52, and 09U089 showed damages of 50, 53, and 54±24.7%, respectively, and genotypes 09U138 and 12U399, showed the lowest result for this trait (0%), which is lower than that of the commercial control (76±24.7%), which undoubtedly indicates resistance to pathogen attack; it was also possible to appreciate that the materials UN36, Andina, UN45, 09U116, 12U347, and 12U368 initially did not show symptoms associated with FoPh, but at the end of the experiment they showed 100% damage which was the highest value.
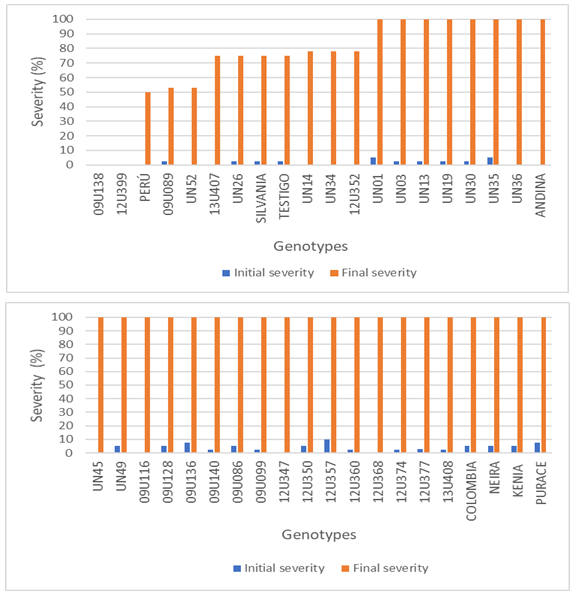
Figure 1 Initial and final severity for the 40 cape gooseberry genotypes inoculated with Fusarium oxysporum f. sp. physalis.
In this regard, Cubedo (2008) and Marín et al. (2018), mention that the diagnosis of F. oxysporum cannot be made immediately since the fungus colonizes the vascular system before the expression of symptoms in the plant, that is, when the disease is detected, the infection is already in advanced stage.
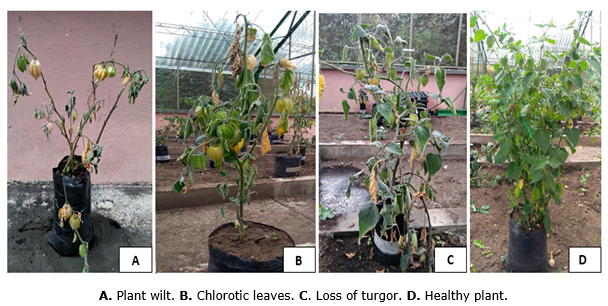
Incidence. The analysis of variance revealed significant differences between the evaluated genotypes (P<0.05), and the Tukey test for comparison of means between genotypes showed that 09U138 and 12U399 had the lowest average for this trait, which was 0, results that differed statistically from the commercial control (75%), followed by Peru and 09U089 with incidences of 25 and 50% respectively, on the other hand the genotypes UN34, 12U352, UN14, 12U360, 09U099, Silvania, 13U407, the commercial control, and UN52 had an average of 75% diseased plants with no statistical differences with each other, even when compared to the control (75%), the rest of the genotypes with the exception of UN26 (81.25%), showed the highest percentage which was 100% (Table 4). When these plants were visually analyzed wilting was observed on the leaves, mainly in the lower part extending to the upper part, causing defoliation and necrosis (Figure 2), therefore they are considered susceptible to attack by F. oxysporum f. sp. physali, and their use is not recommended in soils where there is high inoculum pressure
The results show that these genetic materials lack effective defense mechanisms against the strain used, but their response to other strains is unknown; it should be noted that there are no treatments to cure plants infected by FoPh; besides, this fungus has a wide range of hosts, so measures such as crop rotation are not effective. The use of biological agents does not achieve the desired success because they can be affected by biotic and abiotic factors that make biological control in the field inconsistent
In this research the fungus was able to cause the disease, associated symptoms, and even plant death; in commercial plots, the use of these varieties could cause an increase in inoculum and spread of the pathogen. In relation to the results obtained in genotypes 09U138 and 12U399, it can be inferred that they showed resistance to the attack of FoPh from the first to the last day of the evaluations carried out, since no symptoms associated with the disease such as wilting of basal leaves, loss of turgor, epinasty, chlorosis, prostration of the stalk, and the stem, were observed during the first or second inoculation, chlorosis, stem prostration or necrosis of the stem, or roots (Figure 2 D), this is how these two genotypes become an option for the management of the fungus in our region.
Controlling pathogens that cause vascular wilt is not an easy task, the chemical fungicides that must be applied in the soil around the plant are ineffective especially for fungi such as F. oxysporum, which presents resistance structures that survive for long periods in the soil even in the absence of a host plant (Bani et al.,2018; Chávez et al., 2020; Srinivas et al., 2019).
Regarding resistance, Pulido (2010), Manon et al. (2018), Carmona et al. (2020), and Leitão et al. (2020) state that this measure is the most effective and economically profitable for vascular fusarium in the field. Muñoz et al. (2019), Lamo & Takken (2020), and Leitão et al. (2020) add that the search for genotypes with different degrees of resistance allows a considerable decrease in the frequency of pesticide application, which minimizes the effects on human and environmental health, as well as a direct reduction in production costs. When trying to plan and apply new disease management methods, the objective should be a rational, effective, and safe control at a minimum cost as it is the use of resistant cultivars, many severe fungal diseases, as well as vascular wilt in economically important crops are treated in this way.
Vascular discoloration. Taking into account the scale (Table 3) proposed by Garcés et al. (2017), it was clearly evident that the vascular bundles of genotype 12U374 presented the most severe discoloration in the upper, middle, and lower part, and a reddish brown tone towards the interior of the three portions evaluated corresponding to different heights from the stem to the root (high, middle and low), allowing it to be considered one of the most liable to attack by FoPh (Figure 3), even when compared to the commercial control, which showed discoloration grade 2 (intermediate). Most of the other materials showed intermediate discoloration grade except for 09U086, 09U089, 09U128, 12U357, and UN14, which showed intermediate discoloration grade. 12U357 and UN14 also revealed intermediate necrosis but only in the root portion, in the middle and upper part it was slight. Peru, on the other hand, showed slight discoloration in the three portions evaluated, and the genotypes 09U138 and, 12U399 did not indicate vascular necrosis (Figure 3 and Figure4A: SH=H, SM=M, SL=L); furthermore, the plants did not exhibit external symptoms such as generalized wilting, leaflet loss, growth reduction, or progressive drying, so they could be considered resistant to attack by this strain of Fusarium oxysporum.
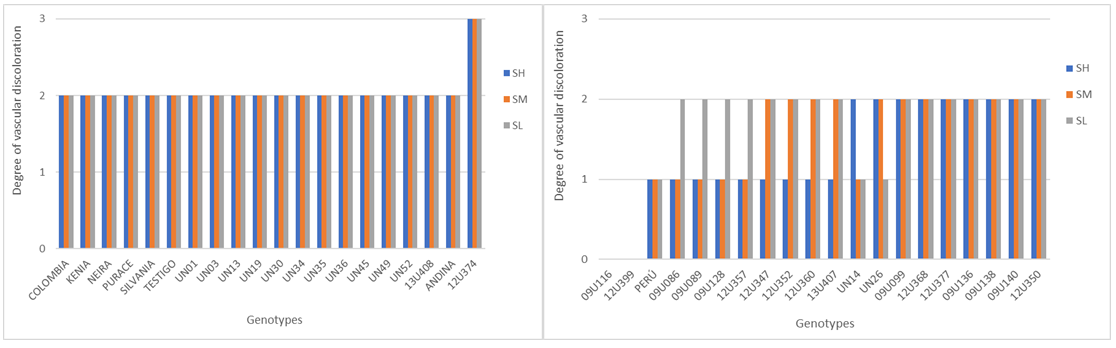
Figure 3 Degree of vascular discoloration of the 40 Goldenberry genotypes in the (H), middle (M), and low (L) of the plant.

Figure 4 Cross section of Goldenberry stem. A. Healthy plant without vascular discoloration B. Plant with discoloration, caused by Fusarium oxysporum f. sp. Physali.
In advanced stages, the roots of the plants show vascular discoloration, necrosis in the stalk base and stem, and discoloration of vascular bundles in addition to necrosis with a pattern of advancing towards the pith (Maurya et al., 2019; Mendoza et al., 2019). Cardona & Castaño (2019), in Solanum lycopersicum describe cross sections with necrotic processes, vascular tissue of dark brown color, being more noticeable at the junction point of the petiole with the stem, similar symptomatology to that presented in genotypes with an intermediate and severe degree of discoloration. In materials such as 12U374, externally, loss of the primary root and grayish-brown lesions at the point of emergence of lateral roots could be observed, as well as adventitious roots above the stem lesion (Figure 4B).
Berruezo (2018) and Patiño (2020) state that vascular necrosis is due to the fact that xylem tracheids become obstructed, preventing communication between the cells of the conducting vessels and the transport of substances, which generates pronounced necrosis; what was observed in genotypes 09U086, 09U089, 09U128, 12U357, and UN14, suggests that it was difficult for the pathogen to colonize the stem of these genotypes, and for this reason its advance occurred only in the area near the inoculation point, thus confirming the ability of this microorganism to mobilize via the vascular system.
Susceptibility index (SSI). To determine the effect of Fusarium oxysporum f. sp. physali (FoPh) on the forty genotypes the susceptibility index was calculated, which made it possible to identify the outstanding materials considering the traits evaluated (plant height, AUDPC, incidence, and vascular discoloration), resulting in a relative value, by which the genotypes could be compared in general. In the analysis carried out, the response of the genotypes was separated into four groups: Very resistant (VR), Resistant (R), Susceptible (S), and Very susceptible (VS).
Figure 5 shows that many of the genotypes evaluated were in the very susceptible category (VS), far from the materials 12U399 and 09U138 which were classified as very resistant (VR), since their susceptibility index was 0; on the other hand, the commercial control reached an SSI of 1 being one of the most susceptible (VS), the above, coincide with that reported by Osorio et al (2017) who by conglomerate analysis classified 09U138 as (VR), this source resistance to FoPh is apparently related with wild-type germplasm. Mayorga et al. (2019) in field tests and soils with high inoculum pressure observed an opposite reaction in which the material 09U138 was (S) to FoPh.

Figure 5 Classification of Goldenberry genotypes according to the susceptibility index MR (Very Resistant), R (Resistant), S (Susceptible), and MS (Very Susceptible). The values were normalized in relation to the commercial control.
The results obtained for 12U399 and 09U138 showed good potential to be incorporated in breeding programs to develop genotypes resistant to FoPh populations. Peru showed a certain degree of tolerance to the fungus indicating an index of 0.5, genotype that together with 12U399 and 09U138 should be evaluated in other regions and against other strains of the pathogen. UN34, 13U407, 09U089, Silvania, UN26, and UN52 indicated an ISS that ranged between 0.83 and 0.96, and that are therefore considered susceptible (S), i.e., F. oxysporum in these plants is capable of penetrating, infecting, and causing symptoms characteristic of the disease and even causing death, which in the field could generate total losses to the farmer. The rest of the genetic materials, as well as the commercial control, are considered very susceptible (VS), although the highest SSI was for UN35 with 1.57. Finally, it should be remembered that the use of resistant genotypes is one of the most appropriate strategies to reduce losses caused by the disease, since production costs are not increased, and the annual application of chemical products is reduced. The resistant genotypes drastically reduce environmental contamination, health, and food risks, and it also guarantees the success of other management tactics.
CONCLUSIONS
Genotypes 09U138 and 12U399 from the first to the last evaluation did not show symptoms such as basal leaf wilt, loss of turgor, epinasty, chlorosis, stem prostration, or necrosis of the stem or roots, so they can be considered resistant to the attack of this strain of F. oxysporum f. sp. physali, however, the results obtained are an advance in the search for resistance of this pathogen and the genotypes should be evaluated in the field, with the same isolation and with other pathogenic strains of the fungus.
Genotypes 09U138 and 12U399 could be used in breeding programs given their resistance to such an important disease as vascular wilt. Although the resistance to Fusarium shown by the other materials did not meet the expectations of this work, it is likely that these genotypes have characteristics of importance for the agro-industrial sector, where their potential should be considered.