INTRODUCTION
Nematodes are of particular presence and importance in coffee plants’ roots, in seedlings, and in adult plantations located in areas with sandy soils. These organisms attack young roots, interfering with the absorption of water and minerals; consequently, the affected plants show symptoms such as nutritional deficiencies in the leaves, defoliation, poor development, and low production. In cases of high severity, and after drought stress, infected plants wilt and die. The most frequent nematodes in Colombia in coffee roots belong to the genus Meloidogyne, being Meloidogyne incognita (Kofoid & White, 1919) Chitwood (1949) and M. javanica (Treub, 1885) Chitwood (1949) complex (root-knot nematode) the most damaging (Rivillas, 2003).
In coffee crops, nematodes management has been directed to the use of preventive measures such as planting coffee plants free of root-knot nematodes from the seedling stage. In the conditions of coffee seedlings, it is recommended to use soil and organic matter free of nematodes and to disinfect work tools to reduce the nematode spread, among others (Rivillas, 2003).
Genetic resistance is another important aspect in the nematode management as the species Coffea dewevrei, C. canephora and C. congensis are the most resistant to the attack of Meloidogyne spp (Rivillas, 2003).
Regarding chemical control, the recommendation is to apply nematicides with systemic action, before or during planting; however, these products are highly toxic to human health and the environment. For this reason, many nematicides are being progressively banned, and their use in plant protection has been restricted. The indiscriminate use of these nematicides and other toxic chemicals not only causes high soil pollution but also generates an increase in crop production costs (Ali et al 2021).
Ali et al. (2021) and Mendoza (2021) mention that current management strategies are directed towards the use of resistant varieties, the application of beneficial microorganisms in biological control, the incorporation of biostimulants, botanical pesticides, synthetic products, cultural practices such as planting times, crop rotation, nutrition, and soil sterilization.
The development of alternatives such as the use of beneficial microorganisms for the bio regulation of pathogenic agents becomes a very significant tool to reduce the damage caused by phytopathogenic nematodes (Cardona & Leguizamón, 1997; Anastasiadis et al., 2008; Collange et al., 2011; Monjil & Ahmed, 2017; Gutiérrez, 2020; Kumar & Arthurs 2021; Ahmed et al., 2022; Karabörklü et al., 2022).
Biological control has been one of the most widely studied topics, and some fungi are identified and classified according to their nematophagous properties, which include trapping, endoparasitism, toxin production, and egg parasites. This last group includes Paecilomyces lilacinus, considered the most successful fungus in the nematodes control of the Meloidogyne genus (Collange et al., 2011; Gutiérrez, 2020; Moreno et al., 2020).
Research on coffee cultivation shows that the Caturra variety is more susceptible than the Colombia and Castillo varieties to the nematodes attack (Rivillas, 2003). This aspect is highly relevant when carrying out studies related to the damage and management caused by Meloidogyne incognita and M. javanica complex in these varieties.
In other studies carried out with nematode biological management in coffee, an efficacy of P. lilacinus, Verticillium chlamydosporium, Beauveria bassiana, Metarhizium anisopliae and Trichoderma spp was shown in the Meloidogyne bio regulation (Cardona & Leguizamón, 1997; Giraldo et al., 1998; Hincapie & Leguizamón, 1999; Leguizamón & Padilla 2001; Bendezu, 2017; Gutiérrez, 2020).
Cardona & Leguizamón (1997) mentions that eggs and the juvenile or female stages of the Meloidogyne genus can be invaded by antagonistic organisms, causing irreversible damage with a significant population decrease of these pathogens in the crop. In this way, one of the most studied microorganisms in coffee and other crops to exert a bioregulatory effect on nematodes has been the fungus Paecilomyces lilacinus, considered a facultative pathogen of nematodes, and recognized as one of the most promising antagonists for the management of root-knot nematode (Rumbos et al., 2006; Ali et al., 2021; Mendoza, 2021).
Based on the above, and with the purpose of evaluating soil-friendly management strategies, the environment, and human health, the MICOSPLAG® biotechnological input (Paecilomyces lilacinus reclassified as Purpureocillium lilacinum, Metarhizium anisopliae, and Beauveria bassiana) and TRICHO-D® (Trichoderma harzianum) were evaluated in the protection of coffee roots against the attack of the Meloidogyne incognita and M. javanica nodulator complex under seedling conditions.
MATERIALS AND METHODS
The experiment was carried out under greenhouse conditions (Cenicafé-Chinchiná) (Altitude 1405m; Latitude 4.983; Length -75.6; coordinates 4º58´57”N - 75º 36.216´W) using coffee seedlings of Caturra variety planted in soil without organic matter addition. The soil used had a sandy loam texture, with a pH of 5.3 and 4.9% of organic matter content. For the experiment, two additional treatments were used where soil + vermicompost (3:1) was used as a traditional agronomic management implemented by coffee growers. Plants were maintained for a period of six months after applying the treatments.
For the knot index evaluation produced by nematodes in coffee plants, the rating scale for Meloidogyne incognita damage in Coffea arabica roots was used. This scale was proposed by the University of North Carolina and modified by Leguizamón in 1995 (Leguizamón & Padilla, 2001).
The traits evaluated were submitted to the Analysis of Variance of a Completely Randomized experimental design. Duncan's mean comparison test was used at the 5% level (P≤ 0.05). The experiment had nine treatments with 16 repetitions per treatment, and the experimental unit was the coffee plant.
The traits evaluated were the degree of infection produced by the nematodes, growth and development of plants (dry weight of the root and aerial part), and persistence of the entomopathogenic fungi of the Micosplag® bioinput in the soil. Table 1, describes the treatments evaluated in the experiment.
Table 1 Description of treatments used in the bio regulation of Meloidogyne incognita and M. javanica complex in coffee roots.
| Treatment | Substrate | Description |
|---|---|---|
| 1 | Soil | Micosplag® (8 DAS) + Nematodes (8 DAM) |
| 2 | Soil | Nematodes (8 DAS) + Micosplag® (8 DAN) |
| 3 | Soil | Tricho-D® (8 DAS) + Nematodes (8 DAT) |
| 4 | Soil | Nematodes (8 DAS) + Tricho-D® (8 DAN) |
| 5 | Soil | Nematodes (8 DAS) + Furadan® (8 DAN) |
| 6 | Soil | Nematodes (8 DAS) |
| 7 | Soil | Absolute control (Sowing without nematodes) |
| 8 | S + V (3:1) | Nematodes (8 DAS) |
| 9 | S + V (3:1) | Absolute control (Sowing without nematodes) |
S + V: Soil + Vermicompost; DAS: Days After Sowing; DAM: Days after Micosplag®; DAN: Days After Nematodes;
DAT: Days After Tricho-D®
The biotechnological inputs evaluated in this experiment were:
Micosplag®, composed of dormant spores of the fungi entomopathogenic Paecilomyces lilacinus (one hundred million spores per gram of commercial product) Metarhizium anisopliae (one million spores per gram of commercial product) y Beauveria bassiana (one million spores per gram of commercial product).
Tricho-D®, composed of dormant spores of the fungi Trichoderma harzianum, strain OBTh 55 - T22 (one hundred million spores per gram of commercial product).
Micosplag® was applied at a concentration of 2g. L-1 of water, using a volume of 20mL/plant at the time of sowing, or at the corresponding time.
Tricho-D® was applied at a concentration of 10g. L-1 of water, using a volume of 20mL/plant at the time of sowing, or at the corresponding time.
As reference control, the product Carbofuran (Furadan®) was used at a dose of 1g/plant eight days after sowing. It is noteworthy that this product is currently banned, and its registration for agricultural use has been cancelled by the Colombian Agricultural Institute - ICA.
For the pathogenic control treatment (Meloidogyne incognita and M. javanica complex) the inoculation was carried out, using 2,500 eggs/plant. The inoculum was previously increased in tomato plants (Solanum lycopersicum L.) Rutgers variety. A modified sodium hypochlorite technique for the extraction of root-knot nematode eggs and larvae from coffee root samples was used in this experiment (Original technique from Hussey & Barker, 1973; modified by Hincapie & Leguizamón, 1999).
Nematodes studies carried out in Cenicafé, prior to this experiment, showed that under field conditions there is a high and varied presence of Meloidogyne incognita and M. javanica in the soil which simultaneously infect and damage the coffee roots. Its population varies depending on soil conditions and the age of the coffee plants (Leguizamón, 1976; Arango, 1977).
Six months later (at the end of the experiment) was evaluated the persistence of fungi P. lilacinus, B. bassiana and M. anisopliae that composed the Micosplag® Bioinput, taking soil samples plus roots of five plants at random corresponding to treatment number 1 which were collected from this experiment and analyzed in the laboratory in order to verify the establishment and permanence of these fungi in the coffee plants rhizosphere.
The soil samples plus roots for the persistence test were sent to the "Control de Bioinsumos" laboratory, which has ICA registry. This laboratory performed the microbiological analysis using dilution tests with sowings in selective media.
RESULTS AND DISCUSSION
Figure 1 shows that infection lowest levels were registered when applying Micosplag® (P. lilacinus, M. anisopliae, and B. bassiana) preventively (eight days before the nematodes inoculation), showing an infection level of 6% in comparison with the pathogenic control in soil (nematodes inoculation), which had an infection level of 51%. In this control, a decrease in root mass and a high nodule formation due to nematode attack were observed (Figure 2).
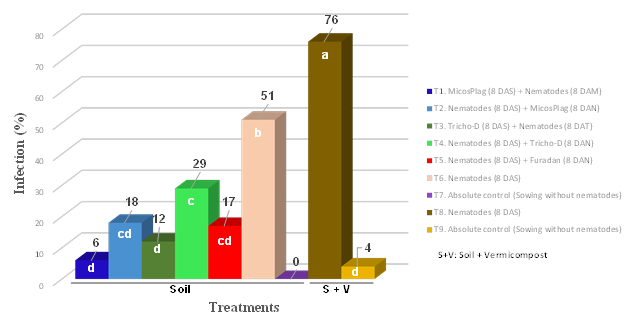
Figure 1 Infection produced by Meloidogyne incognita and M. javanica in coffee plants, six months after applying treatments in greenhouse conditions (Values followed by the same letter are not significantly different, Duncan test at P<0.05).

Figure 2 Root of coffee plant affected by Meloidogyne incognita and M. javanica (corky tap-root, with few roots and presence of nodules).
Figure 2 illustrates the root of a coffee plant corresponding to treatment number 6 (Soil, inoculated nematodes 8 DAS), totally affected by the action of the Meloidogyne incognita and M. javanica complex. It shows root loss, root thickening, corky tap-root, and nodules presence, which are characteristic of this nematodes genus.
With the biotechnological input application as a bioregulator of existing nematode populations, (eight days after nematodes inoculation) a decrease in nematode damage has been achieved, registering an infection level of 18% compared to a level of 51% of the pathogenic control.
The Bioinput preventive application showed lower values of infection in plants than those obtained with the chemical product (Furadan®), which presented an infection level of 17%. Although there were no statistically significant differences between these two treatments, these results highlight not only the infection value which was lower with the Bioinput, but also the is an nematodes management environmentally sustainable.
The Bioinput behavior as a preventive measure focuses on the fungi’s ability to parasitize eggs, preventing the hatching and exit of the larvae; while in its mode of action as a bio regulator of existing nematode populations, it acts by parasitizing juveniles (J2).
Cepeda & Gallegos (2004) point out that Paecilomyces lilacinus is considered an endoparasitic fungus of the root-knot nematode Meloidogyne incognita due to its remarkable adaptability to different types of soil and to its high parasitic potential against eggs and females of the nematodes, causing deformations, ovaries destruction, and limiting egg hatching. The same authors indicate that it has been proven that under slightly acid pH conditions, it produces toxins that affect the nematodes’ nervous system.
Siddiqui & Mahmood (1996) refer to the P. lilacinus efficiency in attacking nematodes due to its ability to colonize the eggs by simple penetration of the eggs’ cuticle, either by mechanical or enzymatic activity. The serine protease produced by this fungus plays a very important role in the nematode egg penetration. Authors also report that P. lilacinus can grow and thrive at temperatures between 15 and 30oC, in a wide pH range, which facilitates the fungi to colonize the soil and to spread rapidly, becoming a highly competitive organism in pathogens bio regulation.
Anastasiadis et al. (2008) under greenhouse conditions, showed that P. lilacinus inoculation, strain 251, and Bacillus firmus, single or associated, provided effective control of J2, eggs, or egg masses of the nematode Meloidogyne spp.
Hashem & Abo-Elyousr (2011) concluded that the biological nematicide P. lilacinus, besides showing a lethal effect on the nematode Meloidogyne incognita, promotes growth in tomato plants.
In coffee crops, Cardona & Leguizamon (1997) in pathogenicity tests under “in vitro” conditions with strain P. lilacinus 9201, was able to obtain the highest infection level (94%) in Meloidogyne females. Giraldo et al. (1998) with strain P. lilacinus 9201 in seedling conditions, produced a controlled production of the nematode Meloidogyne spp., reducing the number of infective juvenile stages between 32 and 49%.
In coffee plants, a regulatory effect of the Meloidogyne nematode by the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae has also been observed. Leguizamón & Padilla (2001) demonstrated, under laboratory conditions, that B. bassiana and M. anisopliae significantly reduced the population of Meloidogyne spp. stages, obtaining the highest reduction with the M. anisopliae fungus. The two fungi showed a high capacity to parasitize nematode eggs. Under seedling conditions, both fungi offered protection to the Caturra variety against Meloidogyne spp. attack. Doses higher than 3g of colonized rice by fungi reduced nematode infection, obtaining a significant increase in the number of plants with grade 1 (healthy plants). Authors mention that the parasitism of these two fungi on Meloidogyne spp. eggs appear to be enzymatic in nature due to cell wall degradation, loss of eggs turgor, and lysis of the J2 stages of the nematode.
The production of enzymes such as chitinase, lipase, and protease, among others from B. bassiana and M. anisopliae, which destroy the Hypothenemus hampei cuticle, has been experimentally demonstrated. The egg wall of Meloidogyne spp. has a composition of lipids, proteins and chitins, which is the reason why the aforementioned enzymes are able to degrade them.
Other authors proved the high antagonistic effect of the fungi Beauveria bassiana, Metarhizium anisopliae, and Paecilomyces lilacinus against the root-knot nematode Meloidogyne incognita, significantly reducing its population and subsequently improving the growth and production of cowpea bean plants (Youssef et al., 2020).
In terms of the protection of roots with Trichoderma harzianum (Tricho-D®), significant statistical differences with the pathogenic control were observed in favor of the biotechnological input. With the preventive application of the Bioinput (eight days before the nematodes inoculation ), an infection level of 12% was obtained in comparison with the pathogenic control that registered a level of 51% (Figure 1).
The fungus Trichoderma spp. proved to have an effective bioregulatory effect against nematodes of the Meloidogyne genus through its toxins and hyphae. Trichoderma harzianum and T. viride are being used in coffee plantations and ornamental plant nurseries, reducing egg hatching and larvae mobility of the genus Meloidogyne (Stefanova, 2007). The same author mentions that the Trichoderma harzianum effectiveness against the root-knot nematode in tomato crops ranges from 52 to 82%. Sahebani & Hadavi (2008) reported that T. harzianum significantly decreased the M. javanica nematode infection in tomato crops due to the high percentage of parasitized eggs by the bio-controller.
Other authors demonstrated the synergism of T. harzianum with other beneficial organisms in the Meloidogyne incognita control. Affokpon et al. (2011) refer to a very important aspect in the pathogens bio regulation, which is that the success of the results depends on the adaptability of the biocontrol agent to climatic and soil conditions. In the research carried out by these authors, it was possible to demonstrate that from 17 isolated strains of Trichoderma spp. from soils with vegetable crops infected with Meloidogyne incognita, five isolates showed a high effect in suppressing the nematode M. incognita in carrot and tomato crops.
Quesada et al. (2019) evaluated the biological action of diverse autochthonous isolates of Trichoderma spp. on Meloidogyne incognita under "in vitro" conditions; results showed that the new isolates reduced the egg masses hatching by 80 to 98%, which led to deformation and parasitism in M. incognita eggs and the embryonic process arrest, some of them in necrotic state. Chitinase activity was also observed in all isolates tested. Kumar & Arthurs (2021) emphasized that Trichoderma spp., besides producing secondary metabolites and enzymes, plays an important role against pathogenic agents; specifically, it has the ability to directly colonize eggs and J2 stages of Meloidogyne spp.
In this research, in coffee plants where P. lilacinus, M. anisopliae, and B. bassiana were applied preventively (eight days before the nematode), an infection level of 6% was reported, and with T. harzianum, also preventively, an infection level of 12% was reported; values that were lower when compared to the chemical product that reported an infection value of 17%. No significant statistical differences were found between these treatments. In the pathogenic agents bio regulation, with the use of beneficial microorganisms, management should be more preventive.
When the bioinputs were applied after nematode inoculation, the infection was lower compared to the pathogenic control (nematode inoculation), but higher for both inputs Micosplag® (18%) and Tricho-D® (29%), compared to preventive application. For treatment eight where nematodes were inoculated in soil plus organic matter, plants were infected at a higher level than that obtained with the pathogenic control in soil (treatment number six), showing an infection value of 76 and 51%, respectively (Figure 1 ). In this aspect, roots of coffee plants planted with the organic matter registered infection levels of 4% caused by native nematodes; an aspect that contributes to increase infection levels in plants that were planted in this substrate and that were inoculated with this complex.
Control plants sown in soil did not show any infection by nematodes. However, coffee plants sown in the substrate that had the vermicompost addition were affected in their growing process by a visible phytotoxicity, which produced a high plant defoliation (T8 and T9). This result indicates that special care should be taken when using organic matter since many of them are difficult to biotransform in their totality until they become humified organic matter. When is not possible to obtain adequate humified organic matter this situation can lead not only to the pathogens presence, as occurred in this research, but also to a high production of alcohols and nutrients that can cause imbalances in plants.
Many specialized microorganisms are involved in the decomposition process of plant and crop residues that achieve an organized and adequate bio transformation of these organic matters, without causing damage such as phytotoxicity that can occur in roots and foliage of plants. Figure 3 shows healthy coffee plant roots treated preventively with the biotechnological inputs, roots treated curatively with the chemical, and roots inoculated with the Meloidogyne incognita and M. javanica complex (pathogenic control).

Figure 3 Roots of coffee plants six months after treatments were applied, A. Micosplag® applied before nematodes inoculation (T1), B. Tricho-D® applied before nematodes inoculation (T3), C. Furadan® applied after nematodes inoculation (T5), D. Nematodes inoculated eight days after planting (T6).
Table 2 shows roots and aerial part dry weights of the plants. Statistically significant differences in plant weight were observed, also between plants inoculated with nematodes (Treatments six and eight), and between those treated with P. lilacinus, B. bassiana and M. anisopliae (Treatment one), and the chemical product (Treatment five).
Table 2 Root dry weight (g) and aerial part of coffee plants six months after treatment application.
| Treatments | Substrate | Description | Dry Weight (g) | |
|---|---|---|---|---|
| Root | Aerial | |||
| 1 | Soil | Micosplag® (8 DAS) + Nematodes (8 DDM) | 0.99 bc | 3.58 bc |
| 2 | Soil | Nematodes (8 DAS) + Micosplag® (8 DDN) | 0.98 bcd | 3.88 ab |
| 3 | Soil | Tricho-D® (8 DAS) + Nematodes (8 DDT) | 0.75 cd | 3.24 bc |
| 4 | Soil | Nematodes (8 DAS) + Tricho-D® (8DDN) | 0.80 cd | 3.21 bc |
| 5 | Soil | Nematodes (8 DAS) + Furadan® (8 DDN) | 1.31 ab | 4.0 ab |
| 6 | Soil | Nematodes (8 DAS) | 0.61 d | 2.65 c |
| 7 | Soil | Absolute control (Sowing without nematodes) | 1.56 a | 4.72 a |
| 8 | S + V (3:1) | Nematodes (8 DDS) | 0.24 e | 0.58 d |
| 9 | S + V (3:1) | Absolute control (Sowing without nematodes) | 0.25 e | 1.27 d |
Values followed by the same letter are not significantly different. Duncan test at P<0.05. S + V: Soil + Vermicompost; DAS: Days After Sowing; DAM: Days after Micosplag®; DAN: Days After Nematodes; DAT: Days After Tricho-D®
Plants corresponding to absolute control in soil showed the highest root weight and aerial part compared to the other treatments. Due to the fact that these plants were not infected by nematode attack, they continued to grow and to develop normally; a condition that was not shown by plants inoculated with nematodes.
Plants sown in soil plus organic matter registered the lowest values in dry weights (Table 2) due to the phytotoxicity problem that had occurred and the presence of inoculated nematodes (T8) plus native nematodes in the vermicompost (T9). In soils of volcanic origin that are mixed with organic compounds, it is very common to find the presence of root-knot nematodes. In this work that presence was confirmed by observing the roots with nodulations (T9). These results confirm the damage caused by nematodes on plant growth and development.
In coffee crops, as a consequence of the damage caused by nematode attacks on roots, there is a reduction in plants growth and nutritional deficiencies; defoliation and loss of plant strength are accentuated. In a crop that is in a productive phase, high levels of infection in roots lead to significant decreases in production (Rivillas, 2003).
In research carried out in order to characterize the root-knot nematode Meloidogyne spp. in coffee plants, Puma (2021) points out the fact that the roots infected by the nematode present knots or galls that are easy to recognize with naked eye, affecting the water and nutrients absorption process, causing a delay in plants development, a reduction in yield, and poor quality production.
Regarding the persistence of the entomopathogenic fungi that compose the Micosplag® Bioinput, results showed that the Paecilomyces lilacinus permanence in the coffee plants rhizosphere was high Followed for B. bassiana and M. anisopliae.
Thus, six months after applying the biotechnological input Micosplag® to the soil, the isolation of P. lilacinus was obtained at a concentration of 5 x 103 CFU/g. B. bassiana was isolated at a concentration of 2 x 103 CFU/g and M. anisopliae was isolated at a concentration of 2 x 103 CFU/g (Figure 4).
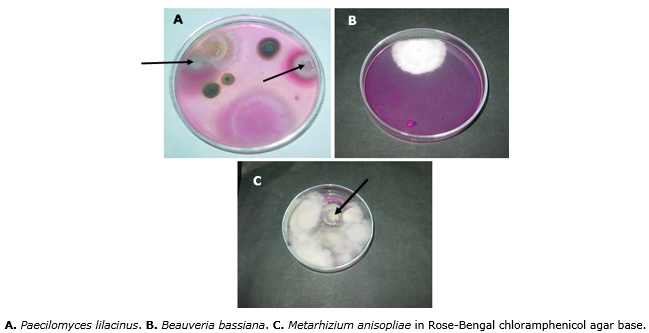
Figure 4 Colonies obtained six months after applying the biotechnological input Micosplag® to the soil.
These results provide reliable information since it is evident that, from the beginning to the end of the experiment (six months later), fungi remained active in soil conditions where the study was carried out, assuring with its presence an antagonistic effect on root-knot nematodes. In addition, the nematodes bio regulation with these fungi is also convenient given their great capacity to remain and multiply in coffee plants rhizosphere.
CONCLUSIONS
Preventive application of Micosplag® biotechnological input (Paecilomyces lilacinus, Metarhizium anisopliae and Beauveria bassiana) in soil had a protective effect of 88.2% on coffee roots against the Meloidogyne incognita and M. javanica complex.
The Bioinput Micosplag® applied preventively had a protective effect on coffee roots against the attack of Meloidogyne incognita and M. javanica complex, superior (64.7% increase in protection) to the one obtained with the chemical product Furadan®.
Preventive application of Tricho-D® biotechnological input (Trichoderma harzianum) in soil had a protective effect of 76% on coffee roots against the Meloidogyne incognita and M. javanica complex.
Preventive application of Tricho-D® bioinput had a higher protective effect on coffee roots against the attack of Meloidogyne incognita and M. javanica complex than the one obtained with the chemical Furadan®, with an increase in protection of 29.4%.
In soil, coffee plants inoculated with the Meloidogyne incognita and M. javanica complex showed a significant decrease in growth and development compared to plants treated preventively with Micosplag® and Tricho-D® Bioinputs, with a decreace of 38% in root dry weight and 26% in aerial dry weight and a decreace of 18.6% in root dry weight and 18.2% in aerial dry weight, respectively.
Six months after the experiment was carried out, the fungi Paecilomyces lilacinus, Metarhizium anisopliae, and Beauveria bassiana were isolated from the soil, showing the high establishment and persistence capacity of these fungi in the rhizosphere of coffee plants.















