INTRODUCTION
The high productivity of crops such as soybean (Glycine max (L.) Merrill) is generally associated with high doses of fertilizers, among other factors. These, in turn, represent a significant portion of the production costs of the crop, in addition to being obtained from a non-renewable source and potential environmental pollutants. The use of plant growth-promoting microorganisms in significant agricultural commodities has been gaining ground in Brazil (Monte et al., 2019). With the intensification of the problems found in chemical resistance breakdown, the use of microorganisms in agriculture is showing promise. It results and is becoming the target of large companies that work with microorganisms for biological control and promoters of plant growth (Ministério da Agricultura, Pecuária e Abastecimento, 2019).
The promotion of plant growth by soil microorganisms can be done through both direct and indirect mechanisms. The direct ones can be the production of hormones or other substances analogous to these, which influence the growth or development of the plant (Zeilinger et al., 2016) or even meeting the required nutritional needs by solubilizing phosphates (Contreras-Cornejo et al., 2016). In contrast, the indirect mechanisms may be through the suppression of pathogens by the action of microorganisms (Saravanakumar et al., 2016; Das et al., 2017; Woo & Pepe, 2018; Mendoza-Mendoza et al., 2018; Monte et al., 2019).
The soil microbiome has the potential to promote plant growth and the tolerance of the plant organism to parasitism of phytopathogens, which may represent a promising sustainable solution to improve agricultural production. Several soil microorganisms, such as fungi, have been reported for their ability to solubilize different forms of inorganic phosphates (Chagas et al., 2016a; Chagas et al., 2017a). Thus, fungi of the Trichoderma genus are found naturally in the soil, which has been the most studied and used as an active ingredient in bio fungicides (Karaoglu et al., 2018; Alekseeva et al., 2019), and they also have activity as promoters of plant growth (Chagas et al., 2017b).
Moreover, Trichoderma is a rhizosphere fungus that promotes plant growth and can stimulate the plant defense system to suppress attacks by phytopathogens (Rubio et al., 2017; Poole et al., 2018). Trichoderma spp.-based bioproducts, such as bio fungicides or biofertilizers, are widely used worldwide (Egamberdieva et al., 2017). The influence of Trichoderma species on plant development is broad; it includes beneficial effects on seed germination, seedling emergence, grain growth, and productivity (Chagas et al., 2016b; Chagas et al., 2017c; Chagas Junior et al., 2019a; Chagas Junior et al., 2019b).
Therefore, producing low-cost inoculants based on plant growth-promoting microorganisms such as Trichoderma spp. is an alternative to reduce the environmental risks caused by the inappropriate and sometimes excessive use of inputs and pesticides. In addition, these fungi increase agricultural production, make the product more competitive and differentiated, and reduce producer costs. Growth-promoting microorganisms can be a great tool for the challenge of modern agriculture in the coming years, which aims to increase sustainable food production with proper environmental protection. Thus, this work aimed to evaluate the efficiency of the product Trichoplus JCO, which contains the active ingredient Trichoderma asperellum (Samuels et al., 1999) as a promoter of soybean plant growth.
MATERIALS AND METHODS
Experiment location. Four independent experiments were carried out in different periods between 2019 and 2020. The experiments were conducted in a greenhouse (photoperiod of approximately 12 hours, average temperature of 26°C and humidity of approximately 85%) at the experimental station of the Federal University of Tocantins, Tocantins, Brazil (11°48'29"S, 48°56'39"W, 280m altitude). The geographical coordinates of the experimental station correspond to 11°43’45’’S and 49°04’07’’W, with an average altitude of 280 meters. The local climatic characterization is of humid tropical climate and classified with minor water deficiency (B1wA’a’) with tropical savanna vegetation according to Köppen-Geiger (Peel et al., 2007).
Product, formulation and Trichoderma. The powdered product Trichoplus JCO (JCO Fertilizers) is a product in a new formulation, containing the active ingredient based on Trichoderma asperellum (Concentration of 2x108 CFU g-1), and graphite was used as an inert along with the Trichoderma strain cultivated in the laboratory and the product prepared in a production room with controlled temperature and humidity. The molecular identification of the isolate was: Trichoderma asperellum GJS 04-217, access to GenBank DQ381958 (99% similarity) (Samuels et al., 2010).
Experiments. Experiment 1 was carried out from August to December 2019 in pots with a capacity of 3.8L, filled with soil classified as dystrophic Red-Yellow Latosol (sieved) with a medium texture. The soil was obtained at the UFT Experimental Station at a depth of 0-20cm, with the chemical analysis as shown in Table 1. The granulometric characteristics were 69.7, 7.0 and 23.3% of sand, silt, and clay, respectively (Embrapa - Empresa Brasileira de Pesquisa Agropecuaria, 2011).
Table 1 Chemical and organic matter analysis of soil samples used in the experiments.
| Experiments | pH | P | K | Al3+ | H+Al | Ca2+ | Mg2+ | SB | T | V | OM | ||||
| H2O | mg dm-3 | ---------------cmolc dm-3--------------- | % | % | |||||||||||
| 1 | 5.1 | 5.0 | 9.0 | 0.0 | 1.8 | 0.5 | 0.2 | 0.72 | 2.52 | 29.0 | 1.0 | ||||
| 2 | 5.8 | 5.7 | 19.0 | 0.0 | 1.5 | 2.2 | 1.1 | 3.3 | 4.9 | 69 | 1.5 | ||||
| 3 | 6.5 | 18.8 | 30.0 | 0.0 | 1.2 | 7.1 | 5.6 | 12.7 | 13.9 | 91 | 10.9 | ||||
| 4 | 5.8 | 5.7 | 19.0 | 0.0 | 1.5 | 2.2 | 1.1 | 3.3 | 4.9 | 69 | 1.5 | ||||
Average values of three soil samples collected for each experiment. Chemical attributes of 0-20 cm depth; pH in water - Ratio 1:2,5; P and K - Mehlich 1 extractor; Al3+, Ca2+ and Mg2+ - KCl extractor (1 mol L-1); H + Al - SMP extractor; SB = Sum of exchangeable bases; (T) = Cation Exchange capacity at pH 7.0; V - Base saturation index; and OM = organic matter (oxidation: Na2Cr2O7 4N + H2SO4 10N).
Experiment 2 was carried out from December 2019 to February 2020 in pots with a capacity of 1.7L, filled with soil classified as dystrophic Red-Yellow Latosol (sieved) with a medium texture. The soil was obtained at the UFT Experimental Station at a depth of 0-20cm in an area other than the soil collected for Experiment 1, which presented the following chemical analysis as shown in Table 1. The granulometric characteristics were 69.0, 7.8 and 23.2% sand, silt, and clay, respectively (Embrapa, 2011).
Experiment 3 was carried out from February to April 2020 in pots with a capacity of 3.8 L, filled with soil classified as haplic plinthosol. The soil was obtained in lowland areas in Formoso do Araguaia, Tocantins, Brazil (11°47'45" S e 49°31'43" W, 240 meters altitude), at a depth of 0-20 cm, with the chemical analysis as shown in Table 1. The granulometric characteristics were 72.5, 10.0 and 17.5% sand, silt, and clay, respectively (Embrapa, 2011).
Experiment 4 was carried out from February to April 2020 in pots with a capacity of 3.8L, filled with soil classified as Red-Yellow Latosol (sieved) with a medium texture. The soil was obtained at the UFT Experimental Station at a depth of 0-20 cm in an area different from the soil collected for experiments 1 and 2, with the chemical analysis shown in Table 1. The granulometric characteristics were 79.8, 8.1 and 12.1% of sand, silt, and clay, respectively (Embrapa, 2011).
In all experiments, essential fertilization (N-P-K + micronutrients) was carried out on the soil before planting, corresponding to 250 kg ha-1 of formulation 05-25-15.
Treatments and design of experiments. Four treatments with different doses of TrichoPlus (3, 4, 5, and 6 g per kg of seeds) and absolute control (without inoculation, negative control) were used in experiment 1. The doses used were considered with the amount of graphite used in soybeans seeds, which has the sole objective of reducing the friction of the seeds with the sowing distribution mechanisms. In contrast, two treatments were used in experiment 2, one with the inoculation of TrichoPlus JCO (5g per kg of seeds) and absolute control (without inoculation).
Inoculation was performed by adding the TrichoPlus JCO product to the seeds in a paper bag and mixing. Then, three treatments were used each in experiments 3 and 4, one with the inoculation of TrichoPlus JCO (5g per kg of seeds), one with the inoculation of commercial product based on T. asperellum (positive control), and an absolute control (without inoculation). According to the manufacturer's recommendations, a commercial product at a concentration of 1x1010 CFU g-1 was used for the positive control, a dispersible granular formulation, and a dosage of 100 g per 100 kg of seeds.
The experimental design for the four experiments was entirely randomized, with five treatments and four replicates. In experiments 1, 2 and 3, two evaluations were carried out, and three evaluations were carried out in experiment 4, with four replicates for each evaluation in each experiment. In all experiments, the experimental units were pots with two plants per pot. The soybean seeds were inoculated with the bacterial rhizobia Bradyrhizobium (SEMIA 5079 and SEMIA 5080, concentration of 3x109 CFU mL-1) with a dosage of 400mL (four doses of 100 mL, according to the manufacturer) per 50kg seeds in the four experiments before inoculation with TrichoPlus JCO, to supply the nitrogen needed by plants.
Soybean cultivars and sowing. The soybean cultivar M 9056 RR was used in experiment 1. The soybean cultivar M 8644 IPRO was used in experiment 2. The soybean cultivar M 8349 IPRO was used in experiments 3 and 4. These cultivars were used in the experiments because they are the most commonly used in the region, being acquired in the region itself. Five seeds per pot were sown in all experiments. The emergence occurred on the fourth day after sowing, and thinning was done, leaving two plants per pot. Daily irrigation was performed according to the field capacity of the pots.
Evaluations of soybean plants. In experiment 1, evaluations were made at 25 and 50 days after planting (DAP). For experiment 2, evaluations were made at 15 and 30 DAP. In experiment 3, an evaluation was performed at 40 DAP. For experiment 4, evaluations were performed at 25, 60, and 112 DAP. Height in centimeters, root volume (VR), and biomass were determined using a graduated ruler to measure the height and the root volume in mL by the breaker method. For biomass, the collected material was washed in running water and taken to dry in an oven at 60°C to determine the dry shoot mass (SDM), root dry mass (RDM), and total dry mass (TDM). The relative efficiency (RE) of each treatment was determined using data from SDM, RDM and TDM according to the equation RE = (SDM, RDM, and TDM inoculated with Trichoderma / SDM, RDM, and TDM without inoculation) (100.
The number of nodules (NN), nodules dry mass (NDM), and nitrogen content in the shoot were also determined. SDM at 50 DAP was ground in a knife mill, where a sample was taken to determine the nitrogen content in the shoot by the Kjeldahl method (Malavolta et al., 1997). The nitrogen accumulation in the shoot (NAS) was calculated by multiplying the SDM by the N content. The phosphorus content in the shoot was also determined through nitric-perchloric digestion (Malavolta et al., 1997). The accumulation of P in the tissues of the shoot was determined by multiplying the SDM by its content in the leaf tissue.
Statistical analysis. The data were subjected to analysis of variance (ANAVA) and T- test and the averages were compared using the Tukey test at 5% probability, using the SISVAR software version 5.1 statistical analysis program.
RESULTS AND DISCUSSIONS
Experiment 1. The results with the different doses of Trichoplus showed that there were significant differences (p <0.05) being superior for RV and TDM when compared to the control treatment at 25 days after planting (DAP). Among these, 5g kg-1 was greater (Table 2).
Table 2 Biomass of soybean plants cv. M 9056 RR, at 25 and 50 days after planting, inoculated with different doses of TrichoPlus (Trichoderma asperellum) applied as in seed treatment.
| Treatments | RV cm3 | SDM g | RDM g | TDM g |
| 25 DAP | ||||
| 0 g kg-1 | 1.6 c | 0.39 b | 0.40 b | 0.79 c |
| 3 g kg-1 | 2.2 b | 0.55 a | 0.52 b | 1.07 b |
| 4 g kg-1 | 2.7 b | 0.56 a | 0.63 b | 1.19 b |
| 5 g kg-1 | 4.3 a | 0.51 a | 0.89 a | 1.40 a |
| 6 g kg-1 | 2.6 b | 0.50 a | 0.51 b | 1.01 b |
| CV (%) | 16.6 | 11.3 | 21.2 | 11.5 |
| 50 DAP | ||||
| 0 g kg-1 | 5.5 c | 0.84 c | 1.35 b | 2.19 c |
| 3 g kg-1 | 7.5 a | 1.05 ab | 1.57 a | 2.57 ab |
| 4 g kg-1 | 6.9 ab | 1.08 ab | 1.63 a | 2.71 a |
| 5 g kg-1 | 7.4 a | 1.17 a | 1.64 a | 2.81 a |
| 6 g kg-1 | 6.8 ab | 1.13 a | 1.58 a | 2.64 a |
| CV (%) | 6.6 | 5.3 | 7.2 | 8.5 |
Means followed by the same letter in the column for a DAP do not differ statistically from each other by the Tukey test at the level of 5% probability. CV: Coefficient of Variation. RV, root volume; SDM, shoot dry mass (g); RDM, root dry mass (g); TDM, total dry mass (g).
For the SDM, all treatments with different doses of TrichoPlus were superior to the control. For the RDM, the treatment with the dose of 5g kg-1 was superior to the others. For the evaluation, at 50 DAP, the RV, SDM, RDM, and TDM, the different doses of TrichoPlus were significantly superior in relation to the control.
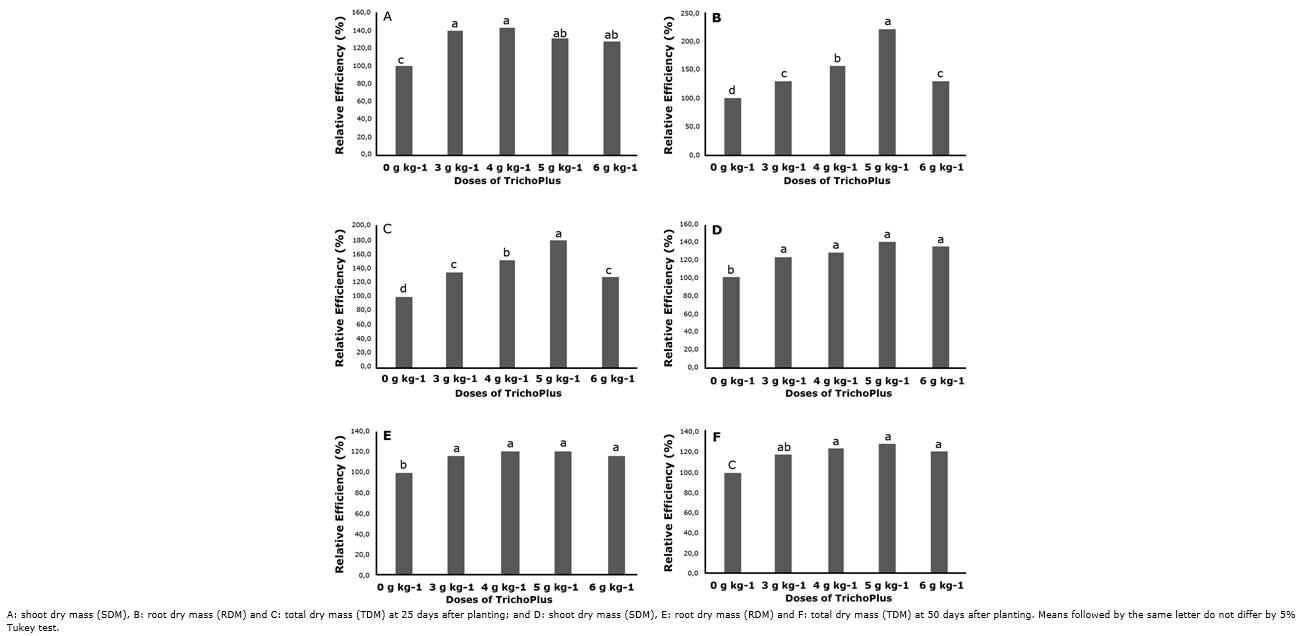
For relative efficiency (RE), at 25 DAP, the results were significant (p<0.05) for treatments with different doses of TrichoPlus, with averages ranging from 28.2 to 43.6% for SDM (Figure 1A), between 22.5 to 57.5% for RDM (Figure 1B), and between 27.8 to 77.2 for TDM (Figure 1C) in relation to absolute control without inoculation.
TrichoPlus (Trichoderma asperellum) concerning to the control without inoculation.Thus, the treatment with 5 g kg-1 was significantly superior to the other doses at 25 DAP (Figure 1B and Figure 1C). On the other hand, at 50 DAP, superior RE values (p<0.05) were observed for treatments with inoculation of TrichoPlus doses concerning the control, with SDM averages between 25.0 to 39.3% (Figure 1D), RDM averages between 16.3 to 20.7% (Figure 1E), and TDM averages between 17.4 to 28.3% (Figure 1F), in relation to absolute control without inoculation.
The NN was significant (p <0.05) in treatments with 4, 5, and 6 g kg-1. For NDM, all treatments with different doses of TrichoPlus were superior (p <0.05) compared to the control (Table 3). In addition, the Nitrogen (N) content and NAS were significantly superior (p <0.05) for the treatments with TrichoPlus in different doses concerning the control. Moreover, the phosphorus content (P) in shoots was superior (p <0.05) in the treatments with the different doses of TrichoPlus and the increased phosphorus content (%P) ranged from 12.9 to 19.4% compared to the control (Table 3). The PAS was significant (p <0.05) in all treatments involving different doses of TrichoPlus. The PAS (4.33g pot-1) and % PAS (66.5%) were significantly superior at dose 5g kg-1 concerning the control.
Table 3 Nutrient contents in soybean cultivar M 9056 RR at 50 days after planting inoculated with Trichoplus (Trichoderma asperellum).
| NN | NDM | N | NAS | P | %P | PAS | %PAS | |
| 0 g kg-1 | 38.5 b | 234 a | 14.1 b | 11.8 c | 3.1 b | 100 c | 2.60 c | 100 c |
| 3 g kg-1 | 45.0 ab | 267 a | 17.8 a | 18.7 ab | 3.5 a | 112.9 ab | 3.68 b | 141.5 b |
| 4 g kg-1 | 48.0 a | 260 a | 18.0 a | 19.4 a | 3.7 a | 119.4 a | 3.99 ab | 153.5 ab |
| 5 g kg-1 | 50.3 a | 281 a | 18.9 a | 22.1 a | 3.7 a | 119.4 a | 4.33 a | 166.5 a |
| 6 g kg-1 | 49.5 a | 270 a | 17.7 a | 20.0 a | 3.6 a | 116.1 a | 4.07 ab | 156.5 ab |
| CV (%) | 11.6 | 11.5 | 10.2 | 11.0 | 9.9 | 9.1 | 10.8 | 10.1 |
Means followed by the same letter do not differ statistically by the Tukey test at 5% probability. CV: Coefficient of Variation. NN, number of nodules; NDM, dry nodule mass (g); N, nitrogen content (g kg.pot-1); NAS, nitrogen accumulation in the shoot (g pot-1); P, phosphorus content in the shoot (g kg-1), %P, phosphorus content increase; PAS, phosphorus accumulation in the shoot (g pot-1); %PAS, increase of phosphorus accumulation in the shoot.
Experiment 2. The evaluations at 15 and 30 DAP showed that the treatment with TrichoPlus inoculation was superior to control for all biomass parameters (SDM, RDM, and TDM) (Table 4). Likewise, all data obtained from the RE of the biomass analyzes were significant (p<0.05) for the treatment with TrichoPlus inoculation for both 15 and 30 DAP (Figure 2).
The SDM, RDM, and TDM averages were 64.1, 150, and 91.5% superior to the control, respectively, at 15 DAP. These averages were also 91, 49.6, and 102.2% superior to the control, respectively, at 30 DAP.
Table 4 Biomass of soybean plants cv. 8644 IPRO, at 15 and 30 days after planting, inoculated by TrichoPlus (Trichoderma asperellum).
| Treatments | SDM | RDM | TDM | |
| 15 DAP | ||||
| TrichoPlus | 1.05 a | 0.75 a | 1.80 a | |
| Control | 0.64 b | 0.30 b | 0.94 b | |
| CV (%) | 19.2 | 23.4 | 17.8 | |
| 30 DAP | ||||
| TrichoPlus | 2.77 a | 1.69 a | 3.66 a | |
| Control | 1.45 b | 1.13 b | 1.81 b | |
| CV (%) | 18.3 | 17.9 | 13.9 | |
Means followed by the same letter in the column for a DAP do not differ statistically from each other by the Tukey test at 5% probability. CV: Coefficient of Variation. SDM, shoot dry mass (g); RDM, root dry mass (g); TDM, total dry mass (g).
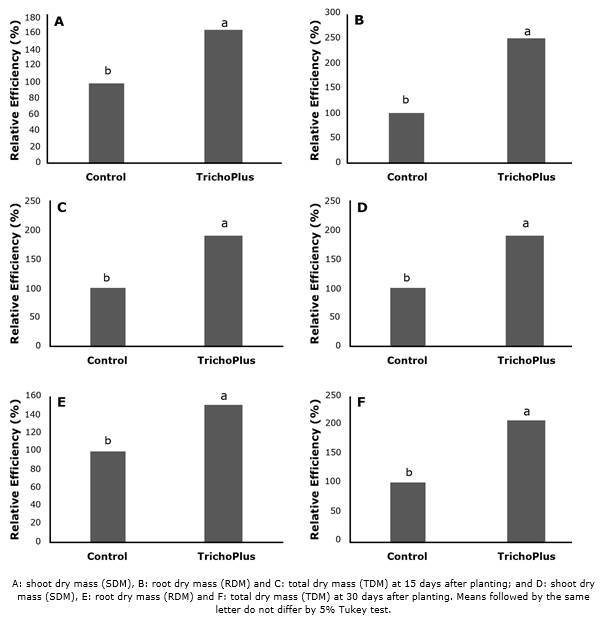
Figure 2 Relative efficiency of soybean plants cv. 8644 IPRO inoculated with TrichoPlus (Trichoderma asperelum) compared to the control without inoculation.
Experiment 3. The treatment with Trichoplus was significantly superior (p<0.05) for SDM, RDM, and TDM concerning the positive control (commercial product) and the absolute control (without inoculation) (Table 5). Otherwise, NN and NDM had no significant differences between treatments.
Table 5 Biomass and nutrient contents in soybean plants cv. 8349 IPRO inoculated with TrichoPlus (Trichoderma asperellum) at 40 days after planting.
| Treatments | SDM | RDM | TDM | NN | NDM | N | NAS |
| TrichoPlus | 3.95 a | 3.55 a | 7.50 a | 28.5 a | 214 a | 15.9 a | 62.8 a |
| Positive control | 3.10 b | 2.90 b | 6.00 b | 25.3 a | 210 a | 14.7 a | 45.6 b |
| Absolute control | 3.00 b | 2.95 b | 5.95 b | 26.5 a | 201 a | 11.4 b | 34.2 c |
| CV (%) | 7.5 | 14.5 | 9.1 | 11.6 | 11.5 | 13.2 | 13.1 |
Means followed by the same letter in the column do not differ statistically from each other by the Tukey test at 5% probability. CV: Coefficient of Variation. Shoot dry mass (SDM, g), root dry mass (RDM, g), total dry mass (TDM, g), number of nodules (NN), dry nodule mass (NDM, g), nitrogen content (N, g kg.pot-1) and nitrogen accumulation in the shoot (NAS, g pot-1).
The treatments with TrichoPlus and the positive control were significantly superior in N content to the absolute control. In addition, the NAS was significantly superior (p <0.05) for the treatment with TrichoPlus inoculation concerning the positive control and absolute control treatments.
The relative efficiencies were significant (p<0.05) in the SDM (28.4 and 31.7%), RDM (22 and 20.3%), and TDM (25.3 and 26.1%) for the treatment inoculated with TrichoPlus compared to the positive and absolute control (Figure 3).
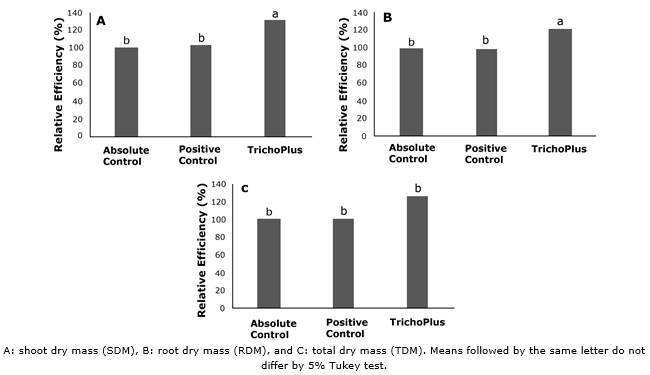
Figure 3 Relative efficiency of soybean plants cv. M 8349 IPRO inoculated with TrichoPlus (Trichoderma asperellum) compared to the positive control (commercial product) and absolute control (without inoculation) at 40 days after planting.
On the other hand, the P content in the shoot of the treatment with TrichoPlus and the positive control was superior (p<0.05) with an increase in %P of 45 and 30%, respectively, compared to the absolute control (Table 6). Then, the PAS and %PAS were superior with treatment inoculated by TrichoPlus, with 92% relative to the absolute control and 57% to the positive control.
Table 6 Nutrient contents in the shoot of soybean plants cv. M 8349 IPRO inoculated with TrichoPlus (Trichoderma asperellum) compared to a positive control (commercial inoculant) and absolute control (without inoculation).
| Treatments | P | %P | PAS | %PAS |
| TrichoPlus | 2.9 a | 145 a | 11.5 a | 192 a |
| Positive control | 2.6 a | 130 a | 8.1 b | 135 b |
| Absolute control | 2.0 b | 100 b | 6.0 bc | 100 c |
| CV (%) | 9.4 | 9.1 | 10.8 | 10.5 |
Means followed by the same letter in the column do not differ statistically from each other by the Tukey test at 5% probability. CV: Coefficient of variation. P, phosphorus content (g kg-1); %P, phosphorus content increase; PAS, phosphorus accumulation in the shoot (g pot-1); %PAS, increase in phosphorus accumulation in the shoot.
Experiment 4. In this experiment, the height in the treatment with TrichoPlus and in the positive control was significantly (p <0.05) than the absolute control at 25 DAP (Table 7). The RV, RDM, and TDM in the treatment with TrichoPlus were significantly superior (p <0.05) to the positive control (commercial product) and the absolute control (without inoculation). Otherwise, the SDM in the treatment with TrichoPlus only differed significantly against the absolute control.
Moreover, the plant heights did not show significant differences between treatments at 60 DAP. The RV in the treatment with TrichoPlus and the positive control was superior (p <0.05) than that in the absolute control. The SDM, RDM, and TDM were superior in the treatment with TrichoPlus concerning the positive control (commercial product) and the absolute control (without inoculation).
Table 7 Biomass of soybean plants cv. M 8349 IPRO inoculated with Trichoplus (Trichoderma asperellum) at 25 and 60 days after planting (DAP).
| Treatments | Height | RV | SDM | RDM | TDM | ||
| 25 DAP | |||||||
| TrichoPlus | 11.53 a | 7.08 a | 0.99 a | 0.80 a | 1.79 a | ||
| Positive control | 10.78 a | 6.15 b | 0.93 ab | 0.69 b | 1.62 b | ||
| Absolute control | 8.45 b | 5.53 c | 0.81 b | 0.60 b | 1.41 c | ||
| CV (%) | 7.29 | 4.73 | 6.99 | 6.24 | 4.01 | ||
| 60 DAP | |||||||
| TrichoPlus | 34.0 a | 12.1 a | 5.23 a | 3.73 a | 8.96 a | ||
| Positive control | 33.5 a | 12.0 a | 4.05 b | 3.20 b | 7.25 b | ||
| Absolute control | 33.0 a | 8.3 b | 3.13 c | 2.38 c | 5.51 c | ||
| CV (%) | 4.12 | 10.22 | 10.87 | 7.86 | 6.48 | ||
Means followed by the same letter in the column for a DAP do not differ statistically from each other by the Tukey test at 5% probability. CV: Coefficient of variation. Height (cm); RV, root volume, SDM, shoot dry mass (g); RDM, root dry mass (g); TDM, total dry mass (g).
At 25 DAP, the relative efficiency values for the treatment with TrichoPlus in SDM was 22.2% and 7.4% higher (p<0.05) than the absolute and positive controls (Figure 4). Likewise, RDM relative efficiency values with TrichoPlus were 33.3 and 18.3% superior (p<0.05) than the absolute control and positive control treatments, respectively. TDM relative efficiency values with TrichoPlus were 27.0 and 12.1% superior (p<0.05) than the absolute and positive controls treatments, respectively. Moreover, SDM (67.1 and 37.7%), RDM (56.7 and 22.2%), and TDM (62.6 and 31.0%) relative efficiency values with TrichoPlus treatment were superior to the absolute control and positive control treatments, respectively, at 60 DAP.
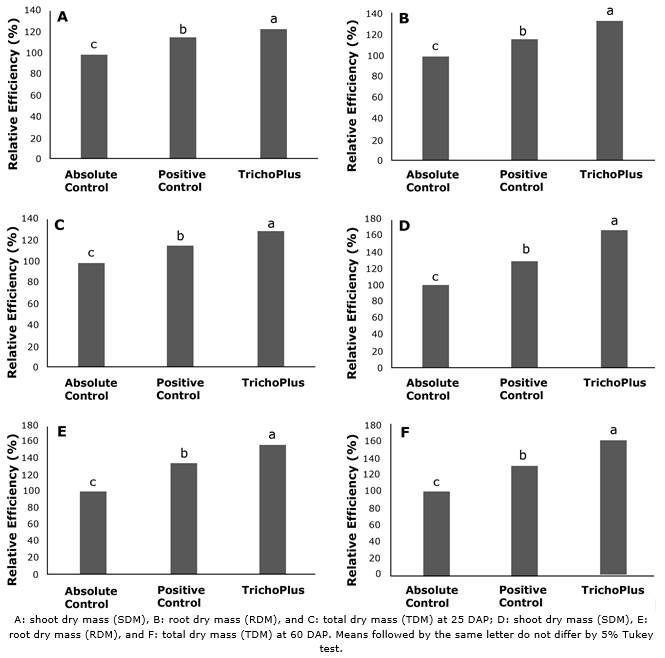
Figure 4 Relative efficiency of soybean plants cv. M 8349 IPRO inoculated with TrichoPlus (Trichoderma asperellum) compared to the positive control (commercial product) and absolute control (without inoculation).
The analysis of the nodules showed that the NN and NDM with TrichoPlus treatment were significantly superior (p<0.05) to the positive and absolute controls (Table 8). The N content had no significant differences between treatments. However, the NAS with TrichoPlus treatment was significant (p<0.05) concerning the positive control and absolute control treatments. On the other hand, the phosphorus (P) content with TrichoPlus treatment and the positive control were superior (p <0.05) with a P content increase (%P) of 36.7 and 26.7%, respectively, compared to absolute control. Thereafter, PAS and %PAS with TrichoPlus treatment were superior (p<0.05) than both controls, which showed an increase in %PAS with TrichoPlus of 128% concerning the absolute control and 64% in relation to positive control.
Table 8 Biomass and nutrient contents in soybean cv. M 8349 IPRO 60 days after planting inoculated with TrichoPlus (Trichoderma asperellum).
| Treatments | NN | NDM | N | NAS | P | %P | PAS | %PAS |
| TrichoPlus | 42.5 a | 314 a | 17.4 a | 91.0 a | 4.1 a | 137 a | 21.4 a | 228 a |
| Positive control | 35.3 b | 260 b | 16.2 ab | 65.6 b | 3.8 a | 127 ab | 15.4 b | 164 b |
| Absolute control | 36.0 b | 271 b | 15.8 ab | 49.5 c | 3.0 b | 100 c | 9.4 c | 100 c |
| CV (%) | 11.7 | 11.5 | 10.5 | 10.2 | 6.6 | 6.3 | 7.5 | 7.2 |
Means followed by the same letter in the column do not differ statistically from each other by the Tukey test at 5% probability. CV: Coefficient of variation. NN, number of nodules; NDM, nodules dry mass (g); N, nitrogen content (g kg.pot-1); NAS, nitrogen accumulation in shoots (g pot-1); P, phosphorus content (g kg-1); %P, phosphorus content increase; PAS, phosphorus accumulation in shoots (g pot-1); %PAS, increase in phosphorus accumulation in shoots.
In the third evaluation, height and grain weight per pot (GWP) obtained with the treatment of TrichoPlus were significant (p <0.05) in relation to both controls (Table 9). The number of pods (NP) and the number of grains (NG) of the treatments with TrichoPlus and positive control were significantly higher (p <0.05) than the absolute control.
Table 9 Height and yield of soybean cv. 8349 IPRO inoculated with TrichoPlus (Trichoderma asperellum) at 112 days after planting.
| Treatments | Height | NI | NP | NG | GWP |
| TrichoPlus | 71.75 a | 19.0 a | 39.9 a | 122.5 a | 14.2 a |
| Positive control | 66.13 b | 18.9 a | 37.8 a | 112.5 a | 11.3 b |
| Absolute control | 60.88 b | 16.6 a | 27.0 b | 82.3 b | 9.2 b |
| CV (%) | 4.59 | 8.18 | 7.55 | 6.25 | 10.71 |
Means followed by the same letter in the column do not differ statistically from each other by the Tukey test at 5% probability. CV: Coefficient of variation. Height (cm); NI, number of internodes (plant-1); NP, number of pods (plant-1); NG, number of grains (plant-1); GWP, grain weight per pot (g pot-1).
All of these effects may be related to the ability of Trichoderma to promote plant growth, as already reported in the literature (Contreras-Cornejo et al., 2015; Li et al., 2015; Contreras-Cornejo et al., 2016; Monte et al., 2019), and the production of auxins and metabolites such as 6PP, which favors the development of the roots, becoming more profound and more vigorous (Zeilinger et al., 2016; Rubio et al., 2017; Mendoza-Mendoza et al., 2018). Several fungi species have been reported to produce auxins in interactions with plants, which are essential hormones that affect growth and development (Contreras-Cornejo et al., 2016; Chagas et al., 2017a). They can also increase the absorption and solubilization of nutrients, such as phosphates, through acidification, redox, chelation, and hydrolysis processes (Li et al., 2015). Likewise, these microorganisms can favor hydrophobic adhesion and the development of absorbent root hairs on the side roots, which increases the water absorption surface and that of some important nutrients, such as nitrogen (Machado et al., 2012; Das et al., 2017; Woo & Pepe, 2018; Mendoza-Mendoza et al., 2018).
The active component of the product TrichoPlus, T. asperellum, showed the production of indole acetic acid, and they also promoted the increase in the production of soybean plant biomass in previous studies, which indicates the relationship between the production of hormones and biomass (Chagas et al., 2017a; Chagas Junior et al., 2019a). Thus, the biomass accumulation in soybean plants in this study can be related to the production of hormones or growth factors, greater efficiency in using nutrients such as P, and increased availability and absorption of this nutrient by plants.
In addition, the fungus T. asperellum can acidify the environment where it is established by the secretion of organic acids such as gluconic, citric, or fumaric acids simultaneously with the synthesis or stimulation of phytohormone production. These acids are the result of carbon source metabolism, mainly glucose, and are capable of solubilizing phosphates, micronutrients, and cations, including iron, manganese, and magnesium, according to Harman et al. (2004).
Thus, the addition of Trichoderma to soils with a scarcity of these cations could result in their solubilization of the available elements, or the addition of poorly soluble natural phosphates as an alternative to phosphorus supplies in the soil, which would increase the production of biomass and productivity of crops (Contreras-Cornejo et al., 2016). Studies have shown that species of Trichoderma spp. can promote increases of up to 300% in plant growth (Brotman et al., 2010). The results obtained in the greenhouse experiments corroborate those obtained by Silva et al. (2011), who evaluated the effect of Trichoderma on the growth of cucumbers and observed a significant increase concerning the control without inoculation of Trichoderma.
In all the experiments, there was an increase in the biomass of the root system, and this increase in the root part is related to the health of the plant provided by the microorganisms. Thus, plants that contain this microorganism associated with their roots or in the rhizosphere tend to have a better ability to survive and absorb nutrients in adverse situations and consequently have a productive advantage over those that do not have Trichoderma in their roots (Contreras-Cornejo et al., 2015; Contreras-Cornejo et al., 2016).
This study's results agree with other studies using different agricultural cultures inoculated with different isolates of Trichoderma. Santos et al. (2010) concluded that the use of Trichoderma spp. provided positive results in increasing the fresh and dry mass of passion fruit plants from cuttings. Carvalho et al. (2011) evaluated the inoculation of Trichoderma isolates in promoting the initial growth of common bean. Jesus et al. (2011) highlighted the potential of T. asperellum as a substrate conditioner to produce coffee seedlings, proving the positive effect on the increase in the root, shoot, and total biomass, as well as the increase in the efficiency of P absorption. Silva et al. (2012) demonstrated that Trichoderma isolates obtained from Amazonian soils increased the biomass of rice plants in the greenhouse, showing potential as growth promoters. Machado et al. (2012) also pointed out that research shows that Trichoderma spp. is efficient, practical, and safe in terms of application methods, biocontrol and plant growth promotion. Likewise, studies have shown the positive effect of Trichoderma on cowpea and soybeans grown in a greenhouse and field conditions (Chagas et al., 2016a; Chagas et al., 2016b; Chagas Junior et al., 2019a; Chagas Junior et al., 2019b).
The results obtained with the application of the inoculant developed with the Trichoderma asperellum strain, which makes up the TrichoPlus product, are promising, especially for the culture under study. The success of the preparation of the inoculant and the inoculation can be credited to its effect as a promoter of plant growth. The benefits of ecological processes performed by fungi of the Trichoderma genus as promoters of plant growth have contributed to achieving sustainability in the agricultural sector (Meyer et al., 2020; Meyer et al., 2022).
The inoculation technology of microorganisms such as the fungus Trichoderma of biotechnological interest in agriculture is a resource of great economic importance, in addition to the contribution to the reduction of use and the consequent impact of agrochemicals, and the global market of biologicals for agriculture, which involves biodefensives, inoculants, biostimulants, and biofertilizers, was estimated at US$ 9.9 billion in 2020 (Borsari & Vieira, 2022).
Sustainable agriculture requires the use of a strategy that increases productivity without harming the environment, opening up new perspectives to contribute to the development of new technologies, methods, and strategies in agribusiness. The processes mediated by microorganisms become essential in preserving and conserving natural resources.
In addition, biological products based on Trichoderma are growing as an alternative to the use of chemical products, being considered to have a low impact on the environment and providing healthier food to the population (Chagas Junior et al., 2018). These products also appear as an important alternative in the biocontrol of phytopathogens due to the different mechanisms of action such as competition (water, air, light, and nutrients); host-parasite association, which can be physical or metabolic with digestion by hydrolytic enzymes, including chitinases, proteases, glucanases, and lipases; and antibiosis, which is the action of a microorganism that acts on another, releasing substances that harm or kill another microorganism (Woo et al., 2014; Oliveira et al., 2022).
The information reported in this study, with the inoculation of TrichoPlus in this formulation, inoculated in soybean seeds, and in a greenhouse, need to be tested in applications under field conditions to prove the efficiency of Trichoderma as a plant growth promoter.
CONCLUSION
The inoculation of TrichoPlus, a Trichoderma asperellum-containing product, in the dosage of 5 g per kg of seed resulted in significantly better values for the biomass accumulation, contents of nitrogen and phosphorus in soybeans. Such findings demonstrate that TrichoPlus can be recommended as an efficient inoculant, promoting the plant growth of soybean plants in the greenhouse.