INTRODUCTION
Pepper (Capsicum annuum L.) is one of the most consumed vegetables in the world (Wang et al., 2018). It is an essential dietary supplement for human health since it contains antioxidant compounds such as carotenes and vitamins C and E (Rodríguez, 2017). In 2020, world pepper production reached 36.136.996 tons (FAOSTAT, 2020). However, germination / seedling emergence is slow and not uniform in pepper, which constitutes a limitation for the economic exploitation of many cultivars (Ureche et al., 2021) .
Pre-germination hydration-dehydration treatments, commonly referred to as seed priming, can reduce the time between sowing and seedling emergence and improve emergence synchronization in the field in several commercial species, e.g., tomatoes (Solanum lycopersicon L.) (Delian et al., 2017), melon (Cucumis melo L.) (Castañares and Bouzo, 2018), Chinese pumpkin (Brassica rapa subsp. Pekinensis) (Yan, 2015), and pepper (Ozbay, 2018). Considering the global interest in organic agriculture, which discourages the use of synthetic substances at all stages of agriculture, there is a growing interest in using organic substances for pre-germination treatments of seeds, i.e., organic priming (Mavi & Atak, 2016).
Plants accumulate protein reserves inside the seeds (Grudkowska & Zagdańska, 2004). Proteases hydrolyze these proteins during seed germination and the beginning of seedling development. Cysteine proteases are the most abundant group responsible for the degradation and mobilization of reserve proteins to promote seed germination. Since bromelain is a cysteine protease, crude bromelain extract from pineapple stems could be a promising alternative for seed priming. This crude extract contains bromelain (90% of total proteases), phosphatases, glucosidases, peroxidases, cellulases, glycoproteins, carbohydrates, and other endopeptidases (Rowan, 2013). However, we found only one study, published in 1994, where bromelain was evaluated to stimulate germination in Rosa multiflora Thunb seeds (Kuska, 1994). This author did not evaluate the priming conditions or the possible causes of the effect generated in the germination by bromelain.
The present study investigated the effects of priming sweet pepper var. Lamuyo seeds with bromelain crude extract on subsequent seedling emergence and growth. The bromelain crude extract was obtained from pineapple (Ananas comosus var. comosus) 'MD-2' stems from crop residues. The objectives of this research were to evaluate: 1) Effect of crude bromelain extract proteolytic activity on pepper sweet seedling's emergence and growth, 2) Effect of crude bromelain extract imbibition time on pepper sweet seedling's emergence and growth, 3) Effect of imbibition temperature on pepper sweet seedling's emergence and growth.
MATERIALS AND METHODS
Plant materials. Sweet pepper seeds var. Lamuyo were obtained from the Ceballos Agro-Industrial Enterprise, Ciego de Ávila, Cuba. Bromelain crude extract was obtained from pineapple (Ananas comosus var. comosus) 'MD-2' stems. Extraction was performed using the procedure described by Hernández et al. (1997) .
Experimental design. The bromelain extract used was obtained using the procedure described by Hernández et al. (1997) and exhibited a total protein content of 2.102 mg.ml-1 (Bradford, 1976), a proteolytic activity of 9.350 U·ml-1 (Anson, 1938), total carbohydrate content of 6.418 mg.ml-1 (Dubois et al., 1956) and a total phenolic content of 0.370 mg.ml-1 (Gurr et al., 1992).
All experiments (1, 2, and 3) were conducted using a completely randomized design (CRD) with three replicates (of n = 100 seeds each) per treatment. No fertilizer, pesticides, or herbicides were used. Two control groups were used for comparative purposes: 1) seeds that were imbibed in deionized water and 2) seeds that were not imbibed. The methodology used to conduct all experiment (1, 2 and 3) were described by Pérez et al. (2021) .
Experiment 1: Effect of crude bromelain extract proteolytic activity on seedling's emergence and growth.
Bromelain solutions with the total proteolytic activity of 0 (distiller water), 3.16, 6.25, 12.5, and 25.0 totalU were prepared, where 1 totalU is defined as the amount of enzyme (in the total volume of extract used) that liberates 1μmol of TCA-soluble, Folin-positive amino acids within 1 minute at pH=6.8 and 37°C, using hemoglobin as a substrate. Besides, a control treatment (untreated seeds) was used. Ten seeds per ml of bromelain solution were used.
Three hundred seeds per treatment were immersed in each solution for 12 hours at 25°C in an incubator (HS62A). Subsequently, the seeds were dried at 25°C for 48 hours until the moisture content was reduced to 7% (equivalent to the initial moisture content), determining the initial and final mass of the seeds using an analytical balance (Sartorius, BL 1 500). As the substrate, the seeds were sown in polystyrene trays using a humus: blond peat moss (1:3) ratio. Seeds were maintained for five days in darkness after they were sown. They were then placed in a greenhouse with natural light, at room temperature, and photoperiod corresponding to the natural diurnal changes (March-April).
Seedling emergence was recorded daily for 14 days; a seedling was considered to have emerged when the emergent plumule measured 0.5 cm. These data were used to calculate the following indicators: emergence percentage (E), time to 50% emergence (TE50), emergence index (EI), mean emergence rate (MER), mean emergence time (MET), and synchronization index (SI), according to Ranal and Santana (2006) . At 35 days in each replicate, the number of true leaves, the length and thickness of the stems, the fresh mass, the dry mass, and the leaf area of 50 plants were measured (Pérez et al., 2021).
Experiment 2: Effect of crude bromelain extract imbibition time on seedling's mergence and growth.
The solution with the proteolytic activity that resulted in the highest seedling emergence and growth in the previous experiment was used to evaluate the effect of imbibition time. The effects of different imbibition times (0 (untreated seeds), 3, 6, 9, 12, and 15 h) were assessed. Ten seeds per ml of extract at 25°C were used. The seeds were subsequently dried and sown as described above. The seedling emergence and growth indicators described in the previous section were calculated.
Experiment 3: Effect of imbibition temperature on seedling's emergence and growth.
The solution with the proteolytic activity and time that resulted in the highest seedling emergence and growth in the previous sections was used to evaluate the effect of temperature in the imbibition. The effects of a range of imbibition temperatures (15, 25, 35, and 45°C) were evaluated using an incubator (HS62A). A control treatment was also used in which the seeds were not embedded in bromelain crude extract (untreated seeds). They were subsequently dried and sown as described above, and the indicators described in the previous section were evaluated. The information from all experiments (1, 2 and 3) described above is summarized in Table 1.
Table 1 Summary information of all experiments (1, 2 and 3) used in this research
| Monofactorial Experiments | Experimental conditions | |||
|---|---|---|---|---|
| Treatments | Proteolytic activity (total U) | Imbibition time (h) | Imbibition temperature (°C) | |
| Experiment 1 | Untreated seeds | - | 0 | - |
| Pa1 | 0 | 12 | 25 ± 1 | |
| Pa2 | 3.16 | 12 | 25 ± 1 | |
| Pa3 | 6.25 | 12 | 25 ± 1 | |
| Pa4 | 12.5 | 12 | 25 ± 1 | |
| Pa5 | 25.0 | 12 | 25 ± 1 | |
| Experiment 2 | Untreated seeds | - | 0 | - |
| It1 | The solution with the best resulted in Experiment 1 | 3 | 25 ± 1 | |
| It2 | 6 | 25 ± 1 | ||
| It3 | 9 | 25 ± 1 | ||
| It4 | 12 | 25 ± 1 | ||
| It5 | 15 | 25 ± 1 | ||
| Experiment 3 | Untreated seeds | - | 0 | - |
| Te1 | The solution with the best resulted in Experiment 1 | The imbibition time with best resulted in Experiment 2 | 15 ± 1 | |
| Te2 | 25 ± 1 | |||
| Te3 | 35 ± 1 | |||
| Te4 | 45 ± 1 | |||
Data analysis. All statistical tests were carried out using SPSS 20.0 software. The Kolmogorov-Smirnov test was used to determined normality of the data while to test the homogeneity of variances, the Levene's test was performed. Parametric tests of analysis of variance (ANOVA) were performed, and means were separated using Duncan's multiple range test (DMRT) (p < 0.05). For statistical treatment, the germination percentage data were arcsine transformed.
RESULTS AND DISCUSSION
Experiment 1: Effect of proteolytic activity from bromelain crude extract on seedling's emergence and growth.
Treatment of sweet pepper seeds with a crude bromelain extract possessing a 6.25 totalU proteolytic activity (Pa3) enhanced seedling emergence relative to control seeds (untreated seeds and Pa1) in terms of the following indicators: E, MET, MER, TE50, SI, and EI (Table 2). However, the results for this beneficial treatment were not significantly different from those obtained for the 12.5 totalU (Pa4) (except for SI) and 25.0 totalU (Pa5) extracts (except for E and SI). Regarding SI, all bromelain treatments improved synchrony significantly (concerning control seeds); however, the 6.25 totalU (Pa3) treatment resulted in the highest SI. Regarding the morphological indicators, the best results (relative to the controls) were also achieved for seeds imbibed in the 6.25 totalU extract (Figure 1).
Table 2 Experiment 1: Effect of bromelain crude extract proteolytic activity during 12 h imbibition at 25℃ on sweet pepper seedling emergence indicators. E: Emergence percentage, MET: Mean emergence time, MER: Mean emergence rate, T50: Time for 50% of emergence, SI: Synchronization index, EI: Emergence index.
| Untreated seeds | Water-dissolved crude bromelain extract (totalU) | ES | |||||
| Distilled Water | 3.16 | 6.25 | 12.5 | 25.0 | |||
| E (%) | 92.50 b* | 90.00 b | 92.50 b | 97.50 a | 95.00 ab | 92.50 b | 3.71 |
| MET (d) | 10.83 c | 10.24 bc | 10.35 bc | 9.51 a | 9.89 ab | 9.97 ab | 0.24 |
| MER (d-1) | 0.0928 c | 0.0961 bc | 0.0967 bc | 0.1052 a | 0.1015 ab | 0.1003 abc | 0.002 |
| T50 (d) | 10.37 c | 9.7 bc | 9.46 ab | 8.7 a | 9.23 ab | 9.27 ab | 0.26 |
| SI | 0.132 c | 0.172 bc | 0.76 b | 0.568 a | 0.296 b | 0.282 b | 0.012 |
| EI (seed day-1) | 0.635 c | 0.705 bc | 0.728 b | 0.825 a | 0.778 ab | 0.751 ab | 0.027 |
*Values with the same letter on a row are not statistically different when compared within indicator categories (One-Way ANOVA, Duncan, P > 0.05, n = 3). For the statistical treatment, emergence percentage data were arcsine transformed. ES: Standard error of the mean.
Abud et al. (2017) reported that proteins represent one of the main reserve components in Capsicum annuum L., seeds. These authors described how, in mature seeds, reserves are deposited both in the embryo and in the endosperm. During the germination of seeds and at the beginning of the seedling development, the reserve proteins are hydrolyzed by proteases and mobilized to supply amino acids, which help the biosynthesis of new proteins and energy generation, which positively influences embryonic development (Keita et al., 1999).
Several authors have described cysteine-protease's involvement in the degradation of accumulated proteins in seeds (Keita et al., 1999; Müntz et al., 2001; Grudkowska and Zagdańska, 2004). For example, 42 proteases are involved during germination in barley seeds, of which 27 are cysteine-proteases (Grudkowska & Zagdańska, 2004). Furthermore, 90% of prolamins degradation in corn and wheat seeds is due to cysteine-protease's activity (Grudkowska & Zagdańska, 2004).
This group of proteases is responsible for endoproteolytic cleavage during the mobilization of storage proteins in the cotyledons of most of the dicotyledonous seeds investigated (Müntz, 1996; Sheokand et al., 2005). However, it should be noted that even though most of the proteolytic enzymes shown to degrade storage proteins during mobilization to date are primarily cysteine-proteases, others are also involved in these processes, like serine-proteases, aspartic acid-proteases, and metalloproteases (Tan‐Wilson & Wilson, 2012).
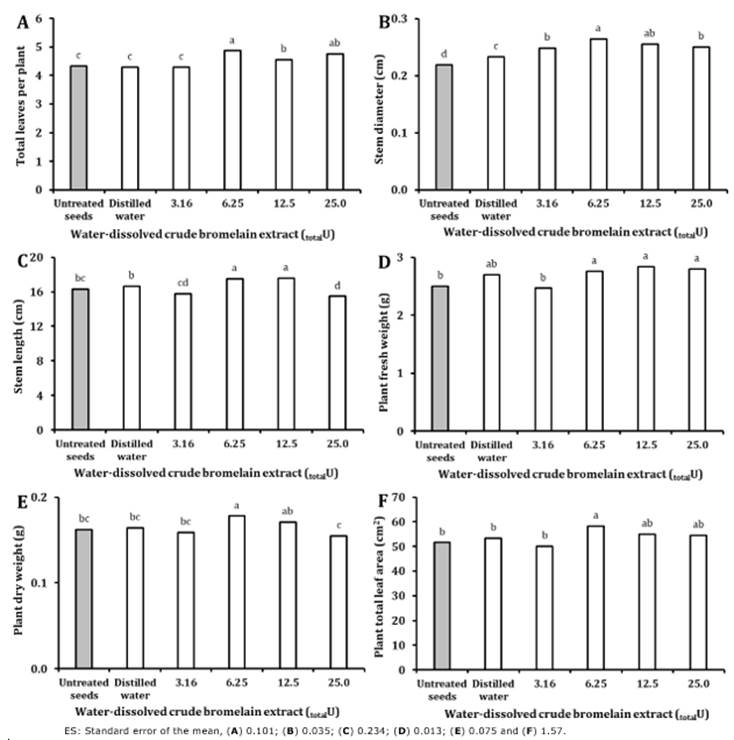
Figure 1 Experiment 1: Effect of bromelain crude extract proteolytic activity during 12 h imbibition at 25℃ on sweet pepper seedling morphological indicators. Values with the same letter for each figure are not statistically different when compared within indicator categories (One-Way ANOVA, Duncan, P > 0.05, n = 50).
Consequently, the positive effects of the bromelain crude extract on seedling emergence in sweet pepper seeds (in terms of MER, EI, MET, TE50, and SI) observed here could be associated with the cysteine-protease (bromelain) found in the extract. This cysteine-protease in promoting the mobilization of protein reserves could have increased the availability of amino acids to the emerging seedling by Pérez et al. (2021). Amino acids are needed for the biosynthesis of enzymes, hormones, proteins, pyrimidines, and purine bases, which contribute to embryo development (Fatima, 2012; Aragão et al., 2015) and subsequent seedling growth as it transitions to the autotrophic stage.
The best results were obtained in terms of the morphological indicators for the treatment Pa3 (6.25total U). This is most likely related to the superior seedling emergence in this treatment; seeds emerged faster, which would have facilitated a more rapid transition to autotrophic growth, photosynthesis, and hence more vigorous plants relative to untreated seeds. Priming has been shown to offer similar benefits in several vegetables (Batool et al., 2015; Behera, 2016) and crop species (Ambika & Balakrishnan, 2015).
While most priming solutions contain osmotica (Zhang et al., 2015; Singh, 2017), an increasing number of studies have investigated the effects of plant extract-based priming solutions (Hala et al., 2017; Kumar et al., 2017; Tiwari et al., 2018). A higher growth rate of seedlings produced from primed seeds has also been associated with improved cell cycle regulation and elongation processes (Lutts et al., 2016). Based on the results described above, all subsequent studies on the effect of imbibition time and temperature were limited to seeds treated with a 6.25 totalU bromelain extract (Pa3).
Experiment 2: Effect of bromelain crude extract imbibition time on seedling emergence and growth.
The imbibition treatment with the best results (relative to the control) in terms of E, MER, MET, TE50, and SI was achieved for seeds imbibed for three hours (It1) in the bromelain extract (Table 3). However, it should be noted that when the seeds were imbibed for 15 h (It5), MET increased significantly relative to the other treatments and the control. Furthermore, for the imbibition during 15 h (It5), MER decreased regarding the rest of the treatments where seeds were embedded in bromelain crude extract (3, 6, 9, and 12 h).
Table 3 Experiment 2: Effect of bromelain crude extract (6.25 totalU) imbibition time at 25℃ on sweet pepper seedling emergence indicators. E: Emergence percentage, MET: Mean emergence time, MER: Mean emergence rate, T50: Time for 50% of emergence, SI: Synchronization index, EI: Emergence index.
| Time of seed imbibition (h) | ES | ||||||
| Untreated seeds | 3 | 6 | 9 | 12 | 15 | ||
| E (%) | 92.50 b* | 97.50 a | 97.50 a | 97.50 a | 97.50 a | 92.00 b | 3.15 |
| MET (d) | 10.73 c | 9.44 a | 9.98 b | 10.18 b | 10.34 b | 11.22 d | 0.15 |
| MER (d-1) | 0.093 c | 0.110 a | 0.100 b | 0.098 b | 0.0967 b | 0.089 c | 0.0015 |
| T50 (d) | 10.04 b | 8.90 a | 9.31 a | 9.57 ab | 9.68 ab | 10.52 c | 0.19 |
| SI | 0.136 c | 0.322 a | 0.234 b | 0.224 b | 0.232 b | 0.190 bc | 0.01 |
| EI (seed day-1) | 0.661 c | 0.789 a | 0.798 a | 0.750 ab | 0.762 ab | 0.704 bc | 0.02 |
*Values with the same letter on a row are not statistically different when compared within indicator categories (One-Way ANOVA, Duncan, P > 0.05, n = 3).
For the statistical treatment, emergence percentage data were arcsine transformed. ES: Standard error of the mean.
In contrast, the most vigorous seedlings were produced by seeds imbibed for three hours (It1). This treatment showed significant differences from the rest of the treatments and the controls regarding the following indicators: length and diameter of the stem, and fresh and dry weight (Figure 2). In relation to plant total leaf area and the number of leaves per plant, the best results were obtained for the 3 (It1), 6 (It2), 9 (It3), and 12 h (It4) of imbibition treatments. However, when seeds were embedded in bromelain crude extract for 15 h (It5), morphological indicators such as stem diameter and fresh mass were reduced with significant differences relative to the other treatments and control (untreated seeds). For this reason, the three h (It1) imbibition time was selected for subsequent studies on the effects of temperature.
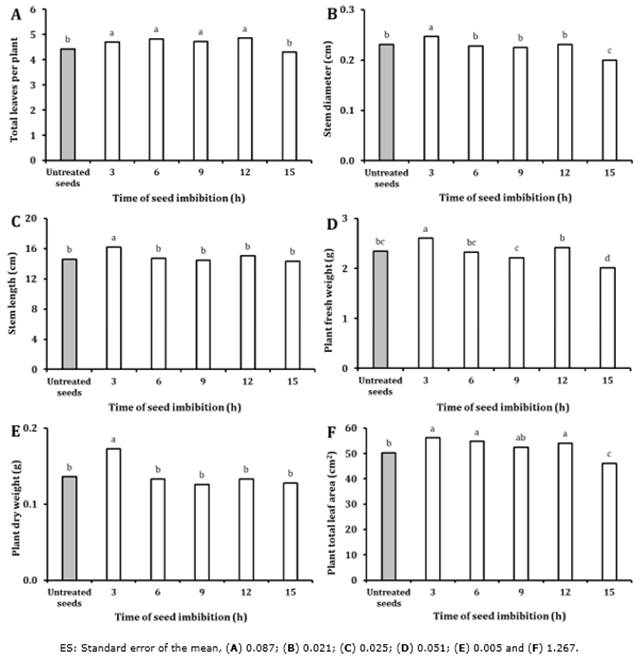
Figure 2 Experiment 2: Effect of bromelain crude extract (6.25 totalU) imbibition time at 25℃ on sweet pepper seedling morphological indicators. Values with the same letter for each figure are not statistically different when compared within indicator categories (One-Way ANOVA, Duncan, P > 0.05, n = 50).
Yambe & Takeno (1992) conducted a study using enzymes from basidiomycete fungi and Trichoderma viride Pers. Ex Gray with activities similar to cellulase, pectinase, glucanase, xylanase, and amylase for seed priming of Rosa multiflora Thunb. These authors reported that the percentage of germination was affected by a prolonged exposure time (60h) of the seeds to these enzymes. In this sense, taking into account the mechanism of action of the enzymes evaluated, this phenomenon was associated with the pericarp that could have been degraded and the seeds.
However, in the present investigation, an extended exposure period (15h) of the pepper seeds to the bromelain extract may allow for maximum extensive proteolysis of seed-stored enzymes required in germination. This may have affected the emergence of the embryo, and consequently, this could have improved seedling emergence time.
Experiment 3: Effect of bromelain crude extract imbibition temperature on seedling emergence and growth.
The best results (relative to the control) for E, MER, MET, TE50, EI, and SI indicators were obtained at 35°C (Te3) (Table 4). On the other hand, when the seeds were imbibed at 45°C (Te4) for three h in the bromelain crude extract, E, MET, TE50, EI, and SI indicators were compromised relative to the other treatments and control (untreated seeds).
Table 4 Experiment 3: Effect of bromelain crude extract (6.25 totalU) imbibition temperature during three h on sweet pepper seedling emergence indicators. E: Emergence percentage, MET: Mean emergence time, MER: Mean emergence rate, T50: Time for 50% of emergence, SI: Synchronization index, EI: Emergence index.
| Temperature of seed imbibition (°C) | ES | |||||
| Untreated seeds | 15 | 25 | 35 | 45 | ||
| E (%) | 92.50 b* | 92.50 b | 97.50 a | 96.66 a | 67.50 c | 2.63 |
| MET (d) | 10.39 b | 9.74 b | 9.76 b | 8.96 a | 11.17 c | 0.18 |
| MER (d-1) | 0.096 c | 0.100 b | 0.100 b | 0.112 a | 0.089 c | 0.001 |
| T50 (d) | 9.68 b | 9.33 b | 9.33 b | 8.16 a | 11.66 c | 0.25 |
| SI | 0.17 b | 0.30 a | 0.27 a | 0.314 a | 0.16 c | 0.01 |
| EI (seed day-1) | 0.74 b | 0.73 b | 0.81 ab | 0.87 a | 0.49 c | 0.03 |
*Values with the same letter on a row are not statistically different when compared within indicator categories (One-Way ANOVA, Duncan, P> 0.05). For the statistical treatment, emergence percentage data were arcsine transformed. ES: Standard error of the mean. 1n =
Seedlings displaying the best morphological indicators (relative to the control) were obtained from seeds exposed to 35°C for three h (Te3). It is worth noting that this treatment also yields the best results for the emergence indicators. Again, this may be due to the rapid seedling emergence associated with three h imbibition at 35°C (Figure 3).
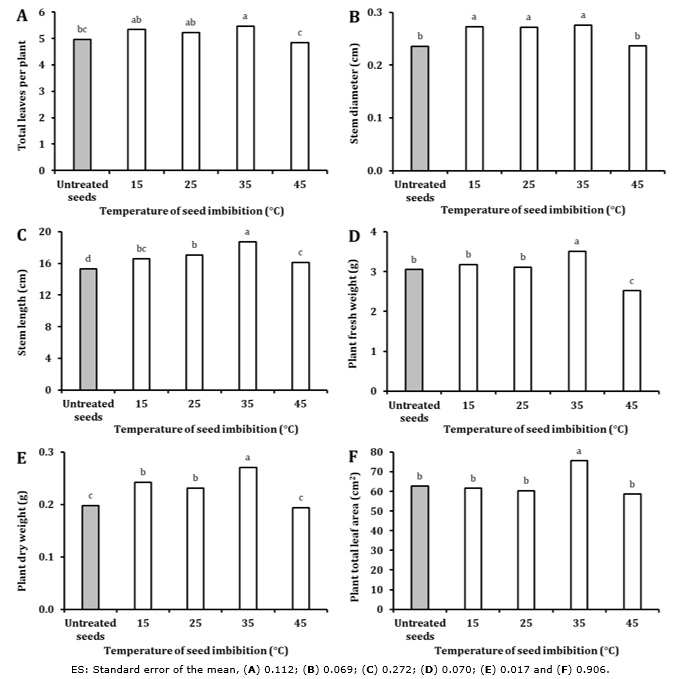
Figure 3 Experiment 3: Effect of bromelain crude extract (6.25 totalU) imbibition temperature during three h on sweet pepper seedling morphological indicators. Values with the same letter for each figure are not statistically different when compared within indicator categories (One-Way ANOVA, Duncan, P > 0.05, n = 50).
The best results obtained at 35°C (Te3) on emergence indicators may be based on the fact that this temperature is closest to the optimum temperature of proteolytic activity for this enzyme (37°C) (Hernández et al., 2005). The breakdown and availability of accumulated protein reserves would have been increased, enhancing proteolytic activity.
On the other hand, according to Shaban (2013) , the maximum seed germination temperature is governed by the temperature at which the denaturation of essential proteins for germination occurs. The maximum temperature for most species ranges between 30°C and 40°C (Yambe & Takeno, 1992). In sweet pepper seeds, the optimum germination temperature is between 20-25°C, and the maximum temperature is 40°C (Carter & Vavrina, 2001). Therefore, exposure to 45°C for three h (Te4), even though this would have constituted phase 1 of germination, may have resulted in the inactivation of enzymes directly involved in seed germination (Dubal et al., 2016). Similarly, exposure to high temperatures (41°C) in corn seeds decreased embryonic protein synthesis, which was attributed to decreased enzyme activity (Rilkey, 1981).
CONCLUSIONS
Pre-germinative treatment with bromelain crude extract of 6.25 totalU proteolytic activity for three h and at 35°C improved seedling emergence and growth in sweet pepper seedlings.This result offers an organic priming alternative for the seeds of this species, probably because bromelain accelerated germination through a greater mobilization of protein reserves.















