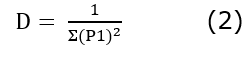INTRODUCTION
Arbuscular mycorrhizae (AM) are found in more than 90% of the roots of plant species, from hepatic plants to angiosperms (Lanfranco et al., 2017). These microorganisms grow especially in soils with a high mineral nitrogen concentration and low available phosphorus content (Urcelay et al., 2019). The symbiosis with these fungi increases the tolerance of plants to environmental stress (Díaz et al., 2013). In addition, plant growth is influenced by the optimization of mineral nutrition and water absorption (Bertolini et al., 2018). AM symbiosis also stimulates the synthesis of secondary metabolites in leaves, fruits, roots, or bark that are used as phytotherapeutic resources (Gianinazzi et al., 2010).
Amazonian soils have low natural fertility, characterized by low levels of organic matter and nutrients, as well as high acidity. Due to these complex edaphic conditions, plant communities have developed associations with arbuscular mycorrhizae fungi (AMF). The genus Glomus is the most common in the Colombian Amazon soils, representing about 50% of the AMF community. This is followed by the genera Acaulospora and Gigaspora (Peña-Venegas et al., 2007).
Both the species richness and the composition of AMF communities depend on the host plant, climate, soil conditions, and nutritional status (Baltruschat et al., 2019). According to Vieira et al. (2019), the diversity of associations between the mycorrhizal fungi and its host has different effects on plant growth under specific ecological conditions. Therefore, studying arbuscular mycorrhizae in Amazonian soils can contribute to understanding soil-biological conditions that affect the growth and development of cat´s claw species.
Uncaria guianensis and U. tomentosa are recognized for their cultural and economic importance. These species are a phytotherapeutic resource widely used in communities of the Amazon region for the treatment of gastrointestinal diseases, measles, and arthritis (Garzón, 2019). Moreover, there is a commercial interest in their pharmacological properties, such as immunomodulatory, antiproliferative, anticancer, and antiviral (Lozada-Requena et al., 2015; Mello, 2017). According to Garzón & Franky (2021), Uncaria species do not have a homogeneous distribution in the southern Colombian Amazon and their propagation is favored in intervened areas. Uncaria guianensis presented the highest abundance and distribution of cat´s claw species, located both in highland and flooded areas within Oxisols and Alluvial Entisols. Likewise, Uncaria tomentosa was found only in the highlands within Oxisols.
Arbuscular mycorrhization in cat's claw species has been poorly addressed. So far, the research of Garzón (2015) in Amazonian soils is only known. Glomus and Acaulospora were identified as the most predominant genera in U. tomentosa soils. There are no available studies about arbuscular mycorrhization in Uncaria guianensis, nor data on the community that colonizes the root of these species. Therefore, this research can provide additional information on the AMF communities associated with Uncaria species and contributes to understanding the ecology of the interaction of AM with soil chemistry in the Colombian Amazon region.
MATERIAL AND METHODS
This research was carried out in two study areas with denudation soils in the southern Colombian Amazon. The first one is located at 3°46'38″ South and 70°18'12″ West on the margin of the Amacayacu River. The Tikuna community of San Martín de Amacayacu is located in that area. The second one is located at 3°53′00″ South and 70°11′17″ West, on the banks of the Amazon River. The Tikuna community of Macedonia is located in that area.
Collection of soil and fine roots. Two types of soils were studied for the growth of Uncaria species: Oxisols (located in highlands) and Alluvial Entisols (located in flooded areas). These second ones are more fertile than those from the highlands because they receive sediments from the Andes region through tributary rivers (Murcia et al., 2007).
Considering that the southern Colombian Amazon has a monomodal rainfall regime, a dry period is not easily recognized. However, the two types of soils were collected during the period of non-flooding where is possible to collect soil samples in both study areas. In 38 sampling points where 27 individuals of Uncaria guianensis and 11 U. tomentosa were found, 500 g of rhizospheric soil was collected at a depth of 0-10 cm (5 samples of Oxisols and 22 samples of Alluvial Entisols for U. guianensis and 11 samples of Oxisols for U. tomentosa). Likewise, an average of 10 fine roots were taken at each sampling point (Table 1) by manual separation from soil and packed in airtight plastic bags for transport to the laboratory.
Table 1 Number of root samples by soil type.
| Study area | Soil type | No. of samples | Samples of Uncaria tomentosa | Samples of Uncaria guianensis |
|---|---|---|---|---|
| Macedonia | Oxisols | 7 | 7 | --- |
| Alluvial Entisols | 6 | --- | 6 | |
| San Martín Amacayacu | Oxisols | 9 | 4 | 5 |
| Alluvial Entisols | 16 | --- | 16 | |
| Total samples | 38 | 11 | 27 | |
Laboratory analysis. The collected soil samples were analyzed at the Soil Laboratory at Instituto Agustin Codazzi - IGAC in Bogota. The Physicochemical analysis included analysis of textural class, pH, exchangeable acidity, aluminum saturation, base saturation, total carbon, available phosphorus, cation exchange content (CEC), total bases, and other elements of the exchange complex.
The analysis of arbuscular mycorrhizae was focused on root staining, in order to evaluate mycorrhizal colonization in cat's claw species. Moreover, spore isolation was conducted for the study of the abundance and diversity of mycorrhizal fungi. The fine root samples were stored at 4ᵒC until their arrival at the Microbiology Laboratory at Instituto Amazónico de Investigaciones Científicas - Sinchi in Leticia.
For root staining, the methodology of Phillips & Hayman (1970) was used. Fine roots were immersed in 10% potassium hydroxide (KOH), heated at 90°C for one hour, and washed with water. Subsequently, these roots were placed in 10% peroxide (H2O2) for 20 minutes and the liquid was discarded. Then, 1% hydrochloric acid (HCl) was added for 15 minutes, and washed with water. The roots were stained with 0.05% trypan blue at 90°C for one hour. Finally, the excess dye was removed and the roots were left overnight in lactoglycerin.
Mycorrhizal colonization was determined using the intersection and field plate methodology proposed by Sánchez de Prager et al. (2010). For each sample, five thin roots of 1 cm in length were placed on a slide with lactoglycerin and counted under the microscope at 10X magnification. A value of 1 was assigned to the field that had structures such as hyphae, vesicles, or arbuscules. Finally, 100 fields were counted and the percentage of total colonization was determined, based on the number of colonized fields over the number of total fields.
The technique of Gerdeman & Nicholson (1963) was used for the extraction of the spores present in 10g of soil. Each sample was placed on three sieves with mesh sizes ranging from 500 to 45 micrometers and washed with water so that the particles passed from the coarsest to the finest sieve. These were then placed in falcon tubes, to which 10mL of a 50% sucrose solution was added and centrifuged for 10 minutes at 3,000rpm. The supernatant containing the spores was again placed on the 45-micrometer sieve and washed with water.
These spores were poured into grid petri dishes and the total count was made with the stereoscope. Using a 100μL micropipette, 10-15 spores per sample were taken and placed on slides with lactoglycerin for identification. The AMF catalog of Peña-Venegas et al. (2006) and the online collection of INVAM - International Culture of (Vesicular) Arbuscular Mycorrhizal Fungi (2019) were used for the description of morphological characteristics. In addition, the taxonomic classification of Baltruschat et al. (2019) was used to define the families and genera of arbuscular mycorrhizae.
Soil and root samples of Uncaria species were also collected for molecular analysis of AMF. For this purpose, community DNA was extracted from the samples using PowerSoil extraction kits, and following the supplier's instructions. Approximately 400mg of soil and 200mg of root were processed to extract community DNA from the samples. The DNA obtained was sequenced at Corporación Corpogen in Bogotá, following the methodology proposed by Öpik et al. (2013).
With the aim of determining the virtual taxa (VT) of AMF existing in the sample, the sequences obtained were cleaned following the bioinformatics analysis methodology reported by Peña-Venegas et al. (2019). The virtual taxa obtained were compared with those reported in the MaarjAM database to determine their taxonomic affiliation.
Statistical analysis. Spearman's correlation test was conducted to determine the significant correlation between soil physicochemical composition, spore abundance, and mycorrhizal colonization of Uncaria species roots. Due to the absence of normal distribution between variables, a non-parametric Mann-Whitney test was used to evaluate significant differences: 1) Root colonization between soil types, Uncaria species and among samples of U. guianensis; 2) AMF spore abundance between soil types, Uncaria species and among samples of U. guianensis and 3) abundance of sequence virtual taxa present in soil and roots of U. tomentosa and U. guianensis. Cluster analysis was used to identify groups of the VTX in soils and roots of U. tomentosa and U. guianensis. All statistical analyses were estimated using R Statical Program 3.5.3, and a significance of p ≤0.05 was adopted.
Species richness (S) was calculated with the number of AM species related to Uncaria species for all soil types. Family diversity of AMF was evaluated using Margalef index (Dmg) and Simpson's index (D), Equation 1:
Where S = number of species and N = total number of individuals. Dmg less than 2 indicates low diversity between species and D near 0 indicates high dominance of one species, Equation 2.
In addition, Shannon index (H´) and Pielou's equality index (J´) were conducted for estimating abundance and evenness, Equation 3:
Where Pi = Ni/N. Ni = number of individuals of species I,Equation 4.
Where H´= Shannon index. H´ less than 2 indicates high homogeneity between species and J´ near 0 indicates less evenness between species.
RESULTS AND DISCUSSION
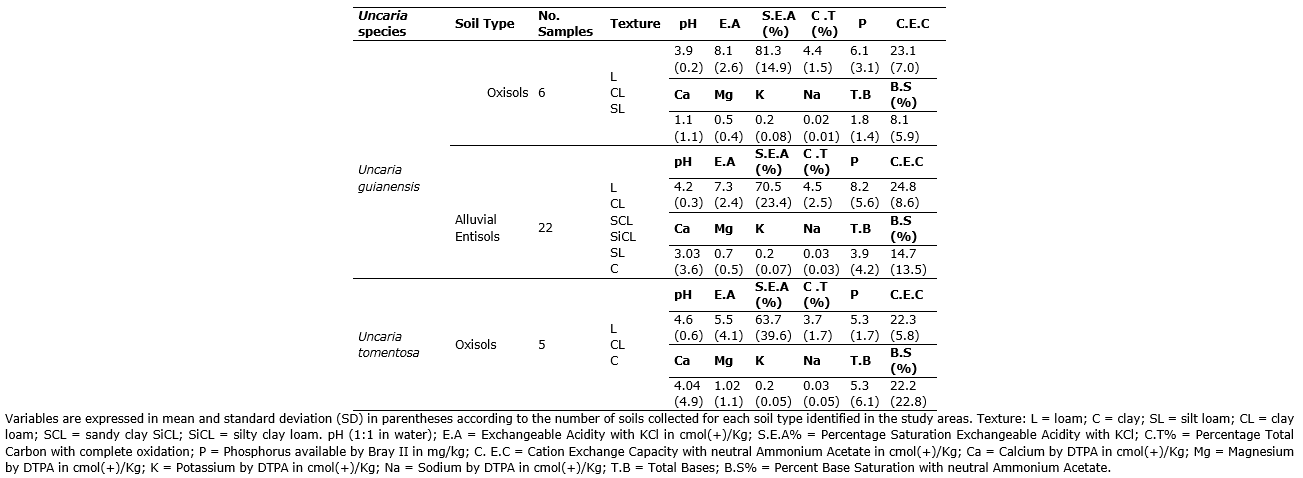
Soil physicochemical composition. Uncaria guianensis is the most widely distributed species, growing in both Alluvial Entisols and Oxisols (Table 2). The analysis of the physicochemical composition of these soils showed that the alluvial Entisols have a greater diversity of textural classes and tend to be less acidic and have a higher concentration of available phosphorus than the Oxisols of the area.
Uncaria tomentosa was found in Oxisols of successional forests. Compared with the results of U. guianensis, the analysis of chemical properties revealed that these soils have higher pH and base saturation, as well as lower available phosphorus and exchangeable aluminum concentrations. These results are consistent with the physicochemical conditions reported by Peña-Venegas et al. (2007) in Amazonian soils.
Mycorrhizal colonization of roots of Uncaria species. Mycorrhizal fungi colonized the roots of Uncaria species via hyphae, vesicles, and arbuscules. The overall percentage of colonization of U. guianensis roots was 68.8%. In Alluvial Entisols, the colonization percentage was 69.2% and 68.5% in Oxisols. Mann-Whitney U test results showed no significant differences between the type of soils (p-value = 0.126) and among samples of U. guianensis (p-value =0.578). Moreover, there was no correlation between AMF root colonization of Uncaria species and chemical variables (Table 3).
Table 3 Spearman´s correlation test for colonization of Uncaria species.
| Variables | U. guianensis | U. tomentosa | |||
|---|---|---|---|---|---|
| Correlation coefficient | Sig. (bilateral) | Correlation coefficient | Sig. (bilateral) | ||
| pH | 0.243 | 0.221 | -0.050 | 0.882 | |
| AI | -0.171 | 0.392 | 0.101 | 0.766 | |
| SAI | -0.361 | 0.064 | -0.003 | 0.914 | |
| C | 0.095 | 0.634 | 0.354 | 0.285 | |
| P | 0.078 | 0.697 | 0.050 | 0.882 | |
| CIC | 0.178 | 0.372 | -0.248 | 0.461 | |
| Ca | 0.292 | 0.139 | 0.027 | 0.935 | |
| Mg | 0.316 | 0.108 | -0.124 | 0.716 | |
| K | 0.213 | 0.285 | -0.220 | 0.514 | |
| Na | 0.223 | 0.262 | 0.058 | 0.865 | |
| BT | 0.353 | 0.071 | 0.013 | 0.967 | |
| SB | 0.299 | 0.129 | 0.124 | 0.716 | |
Significant differences p≤0.05
The average root colonization of Uncaria tomentosa was 71.2%. Mann-Whitney U test results showed non-significant differences in root colonization between Uncaria species (p-value= 0.122). Therefore, Uncaria guianensis and U. tomentosa presented similar root colonization values regardless of the type of soil where they grow. According to Peña-Venegas et al. (2019), species that establish this association tend to be colonized by AMF indistinctly from the species present in the soil.
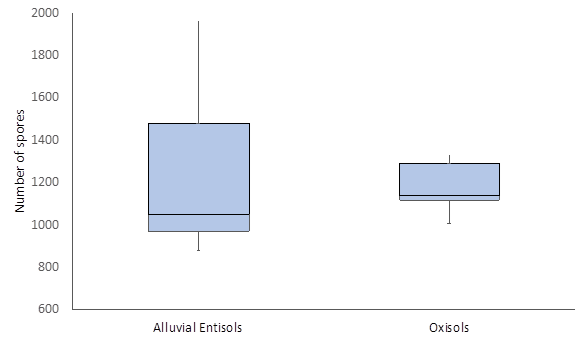
Abundance of arbuscular mycorrhizal fungi. An average abundance of 1.232 AMF spores of soil was found in Alluvial Entisols of U. guianensis. These results showed an asymmetric distribution in a range between 900 and 1,500 spores. In Oxisols, an average of 1,172 spores were found (Figure 1). Likewise, the values presented a smaller dispersion, with a range of 1,100 to 1,300 spores. Mann-Whitney U test results showed non-significant differences (p-value = 0.559) when comparing spore abundance among U. guianensis samples.
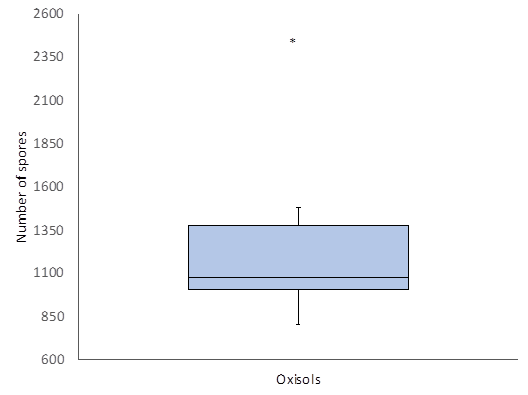
An average abundance of 1,257 spores was determined for the Oxisols of Uncaria tomentosa. For this kind of soil, an asymmetric distribution was found in an interval of 900 to 1,400 spores. An outlier value corresponding to 2,460 spores was observed in a soil sample obtained from a cultivation area (Figure 2). Mann-Whitney U test results showed non-significant differences between Uncaria species (p-value = 0.935). Likewise, non-significant differences between the abundance of spores in the soil types were observed (p-value = 0.680).
Sporulation of arbuscular mycorrhizal fungi does not respond significantly to variations in the physicochemical composition of Alluvial Entisols and Oxisols. However, it was found that spore abundance is lower in acidic soils. It is known that the increase in pH means a decrease in exchangeable aluminum and cation exchange capacity, which reduces the stress of biological populations in the soil. Therefore, both sporulation and arbuscular mycorrhizal symbiosis are favored as a mechanism to secure a host and survive (Peña-Venegas et al., 2007). Thus, a higher number of AMF spores were collected in less acidic soils than in soils with pH values between 4.07 and 5.53.
In the Colombian Amazon, spore abundance increases in areas of greater intervention (Peña-Venegas et al., 2006). The atypical abundance value (2,460 spores) in U. tomentosa soil can be explained by the location of the sample. This sample was collected in secondary vegetation around a cultivation area, with a greater number of propagules in the soil.
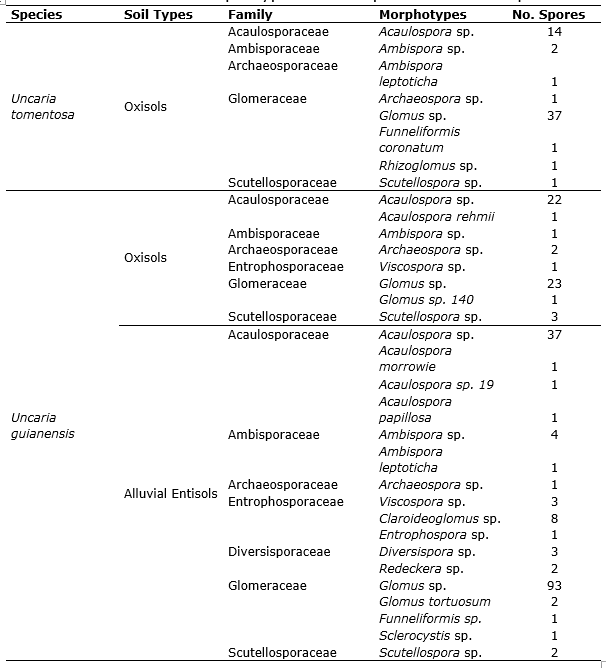
AMF communities in soil types. Through conventional taxonomic analysis of 274 AMF spores obtained directly from the collected soils, 35 AMF morphotypes were determined (Table 4). In rhizospheric soils associated with U. guianensis, were collected 27 spores (9 spores in Oxisols and 18 spores in Alluvial Entisols). In addition, eight spores were collected in rhizospheric soil associated with U. tomentosa. The genera Glomus and Acaulospora were the most frequent in both Oxisols and Alluvial Entisols. However, Ambispora, Archeospora, and Scutellospora were also observed in these soils.
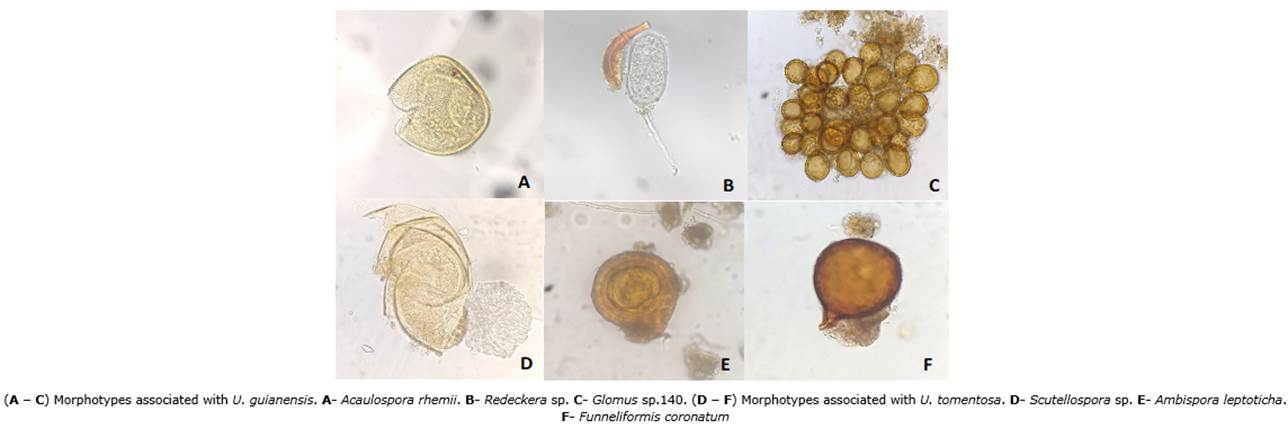
Some differences in the composition of arbuscular mycorrhizal communities were observed in the soil types. There was a higher number of morphotypes in Alluvial Entisols than in Oxisols. The species Glomus tortuosum, Acaulospora morrowie, Acaulospora papillosa, and Acaulospora sp. 19 were found in Alluvial Entisols. Otherwise, the species Acaulospora rehmii and Glomus sp. 140 were observed in Oxisols samples of U. guianensis. The species Funneliformis coronatum were collected in Oxisols samples of U. tomentosa. Additionally, Ambispora leptoticha was the only species present in both soil types (Figure 3).
The AMF morphotypes presented in Uncaria soils were classified into different families, in which Glomeraceae stands out as the most abundant with a total of 160 spores, followed by Acaulosporaceae (77), Entrophosporaceae (13), Ambisporaceae (9), Scutellosporaceae (6), Diversisporaceae (5), and Archaeosporaceae (4). The richness and diversity measurements of these families tend to be homogeneous regardless of the soil type (Table 5).
Table 5 Richness and biodiversity of AMF associated with Uncaria species.
| Indexes | U. tomentosa | U. guianensis | ||
|---|---|---|---|---|
| Oxisols | Oxisols | Alluvial Entisols | ||
| Species Richness (S) | 5 | 6 | 7 | |
| Margalef Index (Dmg) | 0.985 | 1.253 | 1.179 | |
| Simpson´s Index (D) | 0.514 | 0.593 | 0.427 | |
| Pielou´s Index (J¨) | 0.561 | 0.644 | 0.589 | |
| Shannon Index (H¨) | 0.903 | 1.154 | 1.146 | |
Margalef index indicated a low diversity of AMF, especially in the Oxisols associated with U. tomentosa. Simpson's index showed that it is no clear dominance of one family, although the highest richness of AMF species (S) was observed in Alluvial Entisols. Pielou's index indicated a low evenness for mycorrhizal fungi species in both soil types. However, a higher value was estimated in Oxisols associated with U. guianensis. Finally, Shannon index showed a homogeneity between all families presented in soil types.
Molecular analysis of root and soil samples of U. tomentosa yielded 7.306 Glomeromycota sequences, of which 399 were present in soil and 6.907 in roots. For this species, 53 virtual taxa were identified (15 in soils, 13 in roots, and 25 in both samples). Mann-Whitney U test results showed a significant difference (p-value = 0.0297) in the sequence abundance of virtual taxa present in soil and roots. These taxa belong to seven genera of AMF: Glomus, Rhizoglomus, Acaulospora, Gigaspora, Archaeospora, Scutellospora and Claroideoglomus (Table 6). Some species, such as Glomus viscosum and Scutellospora heterogama, were found exclusively in the root, while Gigaspora decipiens was identified in both soil and roots.
Table 6 Virtual Taxa Inventory (VTX) of U. tomentosa.
| Virtual Taxa | Species | Total | Soil | Root |
|---|---|---|---|---|
| GCL-2 | Claroideoglomus sp. | 1 | 0 | 1 |
| LH-Cl01 | Glomus sp. | 1 | 1 | 0 |
| MO-G76 | Glomus sp. | 34 | 31 | 3 |
| VTX00005 | Archaeospora sp. | 20 | 2 | 18 |
| VTX00009 | Archaeospora sp. | 2 | 0 | 2 |
| VTX00012 | Acaulospora sp. | 1 | 1 | 0 |
| VTX00024 | Acaulospora sp. | 8 | 4 | 4 |
| VTX00026 | Acaulospora sp. | 1 | 1 | 0 |
| VTX00028 | Acaulospora sp. | 1 | 1 | 0 |
| VTX00039 | Gigaspora decipiens | 28 | 18 | 10 |
| VTX00063 | Glomus viscosum | 1 | 1 | 0 |
| VTX00069 | Glomus sp. | 1 | 1 | 0 |
| VTX00070 | Glomus sp. | 1 | 1 | 0 |
| VTX00075 | Glomus sp. | 5 | 0 | 5 |
| VTX00076 | Glomus sp. | 35 | 0 | 35 |
| VTX00077 | Glomus sp. | 14 | 0 | 14 |
| VTX00079 | Glomus sp. | 12 | 8 | 4 |
| VTX00080 | Glomus sp. | 132 | 10 | 122 |
| VTX00082 | Glomus sp. | 158 | 0 | 158 |
| VTX00084 | Glomus sp. | 108 | 1 | 107 |
| VTX00089 | Glomus sp. | 12 | 7 | 5 |
| VTX00090 | Rhizoglomus manihotis | 1 | 1 | 0 |
| VTX00092 | Glomus sp. | 377 | 0 | 377 |
| VTX00093 | Glomus sp. | 293 | 61 | 232 |
| VTX00096 | Glomus sp. | 174 | 62 | 112 |
| VTX00103 | Glomus sp. | 16 | 1 | 15 |
| VTX00111 | Glomus sp. | 1813 | 0 | 1813 |
| VTX00112 | Glomus sp. | 16 | 9 | 7 |
| VTX00114 | Glomus sp. | 22 | 2 | 20 |
| VTX00122 | Glomus sp. | 76 | 13 | 63 |
| VTX00126 | Glomus sp. | 154 | 15 | 139 |
| VTX00166 | Glomus sp. | 51 | 45 | 6 |
| VTX00180 | Glomus sp. | 1 | 1 | 0 |
| VTX00186 | Glomus sp. | 3 | 3 | 0 |
| VTX00209 | Glomus sp. | 1 | 1 | 0 |
| VTX00219 | Glomus sp. | 2 | 0 | 2 |
| VTX00227 | Acaulospora sp. | 3 | 3 | 0 |
| VTX00247 | Glomus sp. | 1 | 0 | 1 |
| VTX00248 | Glomus sp. | 786 | 11 | 775 |
| VTX00253 | Glomus sp. | 1 | 0 | 1 |
| VTX00255 | Scutellospora heterogama | 1 | 1 | 0 |
| VTX00269 | Glomus sp. | 1626 | 2 | 1624 |
| VTX00270 | Glomus sp. | 644 | 4 | 640 |
| VTX00280 | Glomus sp. | 17 | 13 | 4 |
| VTX00292 | Glomus sp. | 9 | 9 | 0 |
| VTX00312 | Glomus sp. | 22 | 0 | 22 |
| VTX00318 | Scutellospora sp. | 330 | 164 | 166 |
| VTX00322 | Glomus sp. | 22 | 2 | 20 |
| VTX00361 | Glomus sp. | 19 | 2 | 17 |
| VTX00364 | Glomus sp. | 428 | 1 | 427 |
| VTX00399 | Glomus sp. | 142 | 46 | 96 |
| VTX00403 | Glomus sp. | 6 | 0 | 6 |
| VTX00420 | Glomus sp. | 3 | 3 | 0 |
Values indicate the relative abundance of the species
On the other hand, 19 virtual taxa (VTX) were identified for U. guianensis (8 in soils, 9 in roots, and 2 in both samples). The majority of samples of this species were collected in floodplains, where lower diverse and abundant AM fungal communities could be expected. AM are sensitive to oxygen concentrations, therefore lower levels in floodplains than in uplands could reduce AM spore germination and root colonization (Peña-Venegas et al., 2019). Otherwise, Mann-Whitney U test results showed a non-significant difference (p-value = 0.689) in the sequence abundance of virtual taxa in soil and roots. These taxa belong to three genera of AMF: Glomus, Paraglomus, and Kuklospora (Table 7). Both species, Paraglomus occultum and Paraglomus laccatum, were observed in soil.
Table 7 Virtual Taxa Inventory (VTX) of U. guianensis.
| Virtual Taxa | Species | Total | Soil | Root |
| VTX00080 | Glomus sp. | 51 | 47 | 4 |
| VTX00089 | Glomus sp. | 2 | 0 | 2 |
| VTX00093 | Glomus sp. | 3 | 0 | 3 |
| VTX00096 | Glomus sp. | 29 | 0 | 29 |
| VTX00126 | Glomus sp. | 1 | 1 | 0 |
| VTX00185 | Glomus sp. | 50 | 0 | 50 |
| VTX00204 | Glomus sp. | 16 | 0 | 16 |
| VTX00223 | Glomus sp. | 1 | 1 | 0 |
| VTX00238 | Paraglomus occultum | 3 | 3 | 0 |
| VTX00247 | Glomus sp. | 1 | 0 | 1 |
| VTX00248 | Glomus sp. | 1 | 0 | 1 |
| VTX00249 | Kuklospora sp. | 1 | 0 | 1 |
| VTX00269 | Glomus sp. | 3 | 3 | 0 |
| VTX00280 | Glomus sp. | 1 | 1 | 0 |
| VTX00281 | Paraglomus laccatum | 32 | 32 | 0 |
| VTX00312 | Glomus sp. | 6 | 0 | 6 |
| VTX00369 | Glomus sp. | 1 | 1 | 0 |
| VTX00435 | Paraglomus sp. | 7 | 7 | 0 |
| VTX00444 | Paraglomus sp. | 109 | 58 | 51 |
Values indicate the relative abundance of the species
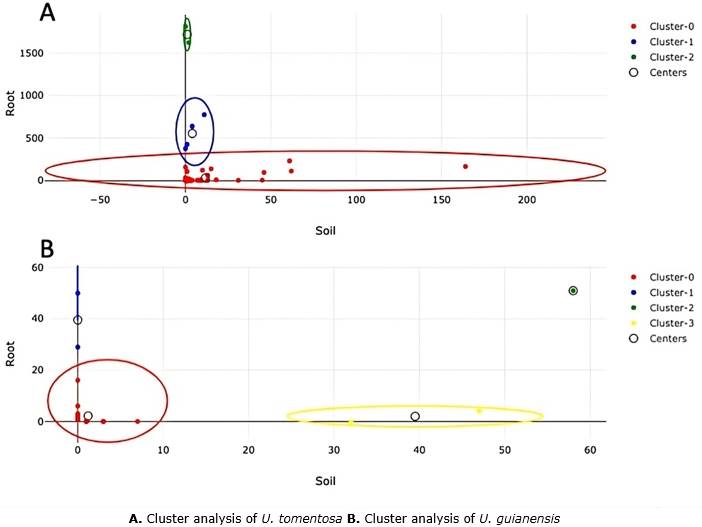
Cluster analysis showed the groups of VTX of U. guianensis and U. tomentosa (Figure 4). The relative abundance of AMF observed in Cluster-1 and Cluster-2 was significantly higher in the roots of U. tomentosa, demonstrating that there is a higher concentration of these fungi in roots than in soil. According to Saks et al. (2014), a higher biomass and DNA concentration of HFMA is found in the root, which is reflected in a lower number of virtual taxa detected in soil. A lower VTX concentration was observed in both soil and root in Cluster-0. Otherwise, U. guianensis presented more clusters than U. tomentosa. In Cluster-0 was observed a higher number of VTX with a similar concentration of AM in roots and soil. This concentration is lower compared with the other clusters, where the concentration of AM is higher in the root (Cluster-1) or in the soil (Cluster-3).
Composition and diversity of AMF communities. The analysis of AMF spores showed differences in the composition of arbuscular mycorrhizal communities in Uncaria species soils. More specific morphotypes were found in Alluvial Entisols (Diversispora, Redeckera Entrophospora, Claroideoglomus, and Sclerocystis) than in Oxisols (Rizhoglomus). The genus Gigaspora is uncommon in Amazonian soils and was not identified by this method; however, Garzón (2015) reported this genus in Oxisols of U. tomentosa.
This identification method is limited because the spores collected did not provide sufficient morphological parameters for easy species distinction. However, all the morphotypes recovered by this method have been reported in the Colombian Amazon by Peña-Venegas et al. (2006). The Pielou´s index showed an equal distribution among the AMF families, suggesting that those who are generalists, such as Glomeraceae or Acaulosporaceae, are not significantly predominant in the soils analyzed.
The results of the prevalence of the Glomeraceae family in this study are similar to those reported by Freitas et al. (2014) and Peña-Venegas et al. (2019) on Amazonian soils. This family is considered a generalist because it is more abundant and diverse in soil types. Therefore, genera belonging to this family can adapt to soils with high acidity, high salinity, or extreme temperatures (Lenoir et al., 2016). In particular, the genus Glomus is the most predominant in soil types because it produces a large number of spores and hyphal fragments. Those structures favor its proliferation in drastic edaphic conditions (Mahmoudiet al., 2019).
Species identified by molecular analysis have been reported by Peña-Venegas et al. (2019) in the Colombian Amazon, and some species such as Gigaspora decipiens, Scutellospora heterogama and Paraglomus occultum have also been mentioned in Brazil (Stürmer & Siqueira, 2008; Novais et al., 2014; Caproni et al., 2018). The presence of species of Gigasporaceae and Acaulosporaceae families mainly in the soil is related to their ability to produce more extraradicular hyphal biomass (Saks et al., 2014). Also, their colonization tends to occur in lower proportion by intraradicular structures such as spores (Moora et al., 2014). In contrast, genera of the family Glomeraceae produce more intraradicular structures (especially hyphal fragments) than extraradicular mycelium, so they extensively colonize the root (Saks et al., 2014).
According to Davison et al. (2020), there is a relationship between the growth form of plant species (cultivated or wild) and their mycorrhizal symbionts. Therefore, the presence of AMF communities in the root may account for the adaptation of the species to its natural environment. Rizhoglomus manihotis and Paraglomus laccatum are commonly associated with cultivated species in agronomic systems (Peña-Venegas et al., 2019), being frequently dominant species in cultivated species such as cassava (Manihot esculenta). However, in Uncaria species, the frequency of those AMF is low. Likewise, the genera Archaeospora and Kuklospora, generally abundant in secondary forests (Martínez-García et al., 2015), are found in low frequencies in the soil and roots of these species. In terms of mycorrhization, U. tomentosa and U. guianensis do not behave as domesticated species. This suggests that their propagation may be more successful through forest enrichment than its propagation in agricultural systems. This kind of system can be considered a foreign environment for Uncaria species.
CONCLUSIONS
Sporulation of arbuscular mycorrhizal does not respond significantly to variations in soil chemical properties. Moreover, root colonization of the Uncaria species is similar regardless of the soil type where they grow. These results indicate that these species act similarly as host plants for arbuscular mycorrhizal fungi in terms of mycorrhization. The AMF community colonizing the roots of U. guianensis and U. tomentosa indicates that these species behave as wild species and not as agricultural species. Therefore, its propagation may be favored through forest enrichment with the species rather than ex situ propagation in agricultural systems. This is the first study that includes molecular analysis of arbuscular mycorrhizal fungi associated with Uncaria species, so it is necessary to advance research in other areas of the Amazon region to expand and contrast the information on the arbuscular mycorrhization of Uncaria guianensis and U. tomentosa.