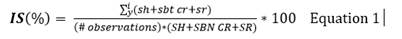INTRODUCTION
In Colombia, purple passion fruit (Passiflora edulis f. edulis Sims) and sweet granadilla fruit (Passiflora ligularis Juss) are economically significant crops, alongside passion fruit (P. edulis f. flavicarpa Degener), as they are exported as fresh and processed fruits. These crops face technological, social, and environmental challenges, along with an increase in foliar and soil-related diseases. In particular, the collar rot disease has amplified the extent of damage within cultivation areas, resulting in crop losses and even crop abandonment. This phenomenon was evidenced by the decline in sweet granadilla production in the Antioquia department during the 1990s. The national contribution of the department dropped from 44.7% to 2.6% due to collar root prevalence (Hoyos-Carvajal & Castillo, 2015).
Collar rot, caused by the fungus Fusarium solani f. sp. passiflorae (Fsp) (Haematonectria haematococca Berk. & Broome) Samuels & Nirenberg (Bueno et al., 2014), is the most significant disease affecting productive passion fruit plants. As plants mature, specific conditions linked to fungal extracellular enzymatic activity (Robledo et al., 2017) facilitate the initiation of colonization and infection in the root system and/or stem base. This triggers the development of characteristic aerial symptoms of the disease, ultimately leading to plant death (Ploetz, 1991; Ploetz, 2005; Porter et al., 2015).
In Colombia, there are few studies conducted on infection systems in passion fruits. Among them, Londoño (2012) evaluated 28 accessions from the passion fruit germplasm bank maintained by AGROSAVIA - Colombian Agricultural Research Corporation, testing two inoculation methods: 1. stem incision and 2. naked root immersion, using a strain of Fusarium oxysporum. Similarly, Ortiz & Hoyos (2016) aimed to standardize several inoculation methodologies for F. solani and F. oxysporum, comparing root immersion techniques with wounded and unwounded roots in four-month-old purple passion fruit plants. They found a high feasibility of disease development with the first technique and associated this phenomenon with the presence of wounds that increased the plant's susceptibility to pathogen invasion.
Ángel et al. (2018) compared several invasive methods, including stem incision, root immersion, stem injection, and screening in modified test tubes. The incision and screening treatments yielded 100% of disease incidence.
On an international level, in Brazil, Fischer et al. (2005) evaluated inoculation methods that included wounds and subsequent inoculation of N. haematococca on passion fruit plants of varying ages. They found that inoculations at the plant's collar led to elevated disease levels, in contrast to root system inoculations. This indicated that the pathogen's infections are more successful if the plant has injuries and in younger plants. Preisigke et al. (2017) assessed disease development in 14 passiflora species, which were inoculated with F. oxysporum through root immersion in a conidial suspension, leading to symptom development and plant death. Other studies evaluated the resistance of P. mucronata Lam against F. oxysporum f., sp. passiflorae and F. solani using the root immersion method, which was effective for selecting resistant rootstocks (Correia et al., 2022). Similar results were reported by Bueno et al. (2014), CEPASS & ASOHOFRUCOL (2010), and Ortiz & Hoyos (2016).
Artificial infection systems constitute a valuable tool in plant disease studies, as they allow researchers to observe and measure pathosystem-specific characteristics with greater precision and ease than under natural conditions. This type of infection systems is needed to better understand the passiflora-F. solani pathosystem, and to develop adecquate experimental tools to test disease control practices or biocontrol agents. They also are necessary for sound epidemiological studies and to develop routine germplasm screening protocols in the search for sources of resistance. Collar rot continues to be a limiting disease affecting passifloras worldwide, and causes severe losses wherever purple passion fruit and sweet granadilla orchards are grown in Colombia. The present study highlights a comprehensive infection-evaluation system which will foster epidemiological and disease control studies of collar rot in the future
MATERIAL AND METHODS
Location. The experiments were conducted from January to March 2017 and from December 2019 to February 2020 at the Laboratorio de Microbiología Agrícola and the greenhouses of AGROSAVIA - Colombian Agricultural Research Corporation, Centro de Investigación Tibaitatá (Mosquera, Cundinamarca), in the Sabana de Occidente province, Cundinamarca department, Colombia; coordinates 4°41'45"N 74°12'12"W, 2516 meters above sea level. During both assessments, the average temperature was 23°C, with a relative humidity of 70%.
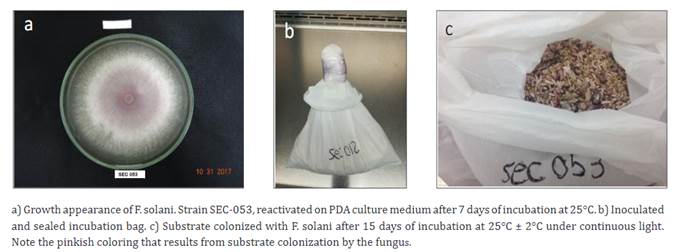
Strain of Fusarium solani f. sp. Passiflorae . We used strains of Fusarium strains SEC-053 (sweet granadilla) and SEC-173 (purple passion fruit) that had been previously characterized morphologically and microscopically on culture media: carnation leaf agar (CLA) and potato dextrose agar (PDA) (Bueno et al., 2014; Porter et al., 2015; Vargas, 1992), and through molecular methods for Fusarium species using ITS-Fu2, ITS-Fs5, and TEF-Fs4 markers (Arif et al., 2012; Bueno et al., 2014). The strains, which had been purified and stored in a working microorganism collection, were reactivated on a synthetic culture medium (PDA) in Petri dishes at a temperature of 25 ± 2°C in darkness for 7 to 10 days (Figure 1a).
Starting from the pure colonies obtained during the strain reactivation, we extracted mycelium from the colony periphery using 5 mm-diameter cork borers (as an alternative method, the colonies can be scrapped with a glass rod by adding 4 mL of sterile water). The culture medium discs with F. solani (or the mycelium and conidial suspension obtained from plate scraping) were used to inoculate sterilized rice substrate, facilitating the extensive propagation of the fungus.
Mass Multiplication of F. solani Inoculum in Rice Grains. The fungus enhancement substrate was prepared using the modified Porter method (Porter et al., 2015). For this, 250 g of rice was weighed and mixed with 120 mL of potato dextrose broth (PDB) in a polypropylene bag resistant to autoclaving to facilitate rapid and uniform fungal growth and colonization. To allow gas exchange and prevent excessive moisture accumulation and subsequent fermentation, the bag was sealed with a cotton plug covered with gauze and sealed with tape.
The bag containing the substrate mixture was autoclaved at 121°C and 15 pounds of pressure for 15 minutes and allowed to rest at room temperature under aseptic conditions for 6 to 12 hours before inoculation with F. solani. The mycelium discs, or fungal suspension, were inoculated into each 250-gram bag of substrate. The bags were then resealed with the plug and tape. This process was carried out in a laminar flow cabinet.
After the inoculation, the bags (Figure 1b) were incubated at 25°C ± 2°C for 12 to 15 days under continuous light, with manual homogenization of the contents every three days. This facilitated the uniform growth of the fungus across the substrate's surface (Figure 1c).
At the end of the incubation period, a conidial count was performed to determine the concentration. For this count, a 1:10 w/v dilution was prepared using 1 g of colonized substrate (Figure 1c) suspended in 9 mL of sterile, distilled water and homogenized using a vortex mixer. An aliquot was collected to perform F. solani conidial counting with a Neubauer chamber.
Plant material. We used commercial ecotype seedlings of sweet granadilla and purple passion in the vegetative state: 12 to 16 weeks old, grown in peat. Planting material for the experiments was provided by Cepass nurseries located at the department of Huila in 2017, and from the Biosystems Center of the Universidad Jorge Tadeo Lozano in Bogotá, Colombia, in 2020. In both cases, seedlings exhibited well developed root and leaf systems. Before inoculation, seedlings of both species were acclimated for a period of 15 to 20 days in a greenhouse with an average temperature of 23°C and a relative humidity of 70%. Plants were watered every week to mitigate stress and deterioration before transplanting them into bags. Plants were closely monitored to minimize losses and maintain uniformity among them.
Inoculation and experimental design. We chose moistened Promix® extra fine peat (1.5L of water per 10L) as the substrate for the inoculation for its aseptic characteristics and the presence of fine, loose particles that offer ease of management. We deemed this substrate optimal for fostering root system development in sweet granadilla plants.
The inoculation protocol was implemented as follows: 150 g of moistened peat was blended with different amounts of inoculum to create three distinct treatments, resulting in final concentrations of 0.5x106, 1x106, and 2x106 conidia per gram. An uninoculated control group (plants without Fsp) was also included for comparative analysis. The experimental design adhered to a randomized complete block pattern with three replications and eight plants per experimental unit for the first trial conducted in 2017. Subsequent evaluations in 2020 followed a similar randomized complete block design, comprising four replications with six plants per experimental unit.
For transplanting, we filled each bag up to 2/3 of its capacity with moistened peat. An orifice was made at the center of the substrate to introduce the seedling. The bag was then filled completely with the mixture of moistened peat and colonized rice substrate. We made sure that the inoculum was evenly distributed and in contact with the base of the seedling stem. Subsequently, a nutrition regimen was implemented twice a week by applying Hoagland and Arnon solutions (Hoadgland & Arnon, 1950). The health status of the plants was continuously monitored.
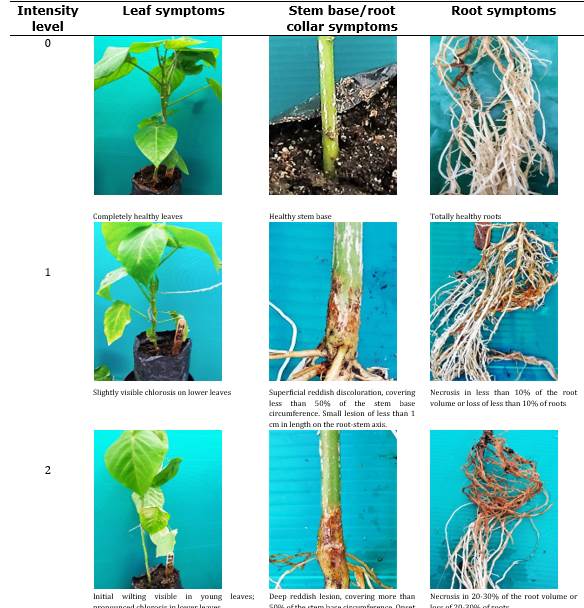
Assessment of Sweet Granadilla and Purple Passion Fruit plant responses to F. solani Infection. Around 10 days after inoculating the sweet granadilla and purple passion fruit plants with F. solani, we started daily monitoring to detect any symptoms. Once initial symptoms appeared, the onset date was recorded, and assessments were conducted every three days to document damage progression in each plant. The description of disease-related damage was carried out using descriptors specifically generated for this purpose (Table 1 and Table 2).
Symptom assessments on foliage (SH) were conducted from the moment of inoculation until plant death. Symptoms on the stem base/root collar (SBT-CR) and roots (SR) were performed at the end of the process (or earlier in the case of plant mortality). Using the collected data, we calculated the disease severity index (DSI) using the following equation 1 (Chiang et al., 2017b).
Where:
sh: value assigned to foliage symptoms for each plant
sbt cr: value assigned to stem base and root collar symptoms for each plant
sr: value assigned to root symptoms for each plant
SH: maximum value on the scale for foliage symptoms
SBT CR: maximum value on the scale for stem base and root collar symptoms
SR: maximum value on the scale for root symptoms.
The plants that reached intensity level 4 in SH underwent a symptom evaluation in the stem base and roots (SBT-CR and SR) (Londoño, 2012). For the assessment of symptoms in the stem base and root collar (SBT-CR), the size of the lesion was estimated based on the coverage achieved. The substrate and roots from different treatments were microbiologically analyzed using PDA and Komada agar (Komada, 1975) at the end of each experiment to isolate the inoculated strains and confirm the presence of the pathogen.
Statistical analysis
To perform the data analysis, we tested the assumptions of normality, independence of errors, and homogeneity of variance using the Shapiro-Wilk, Levene, and Durbin-Watson tests. We employed generalized linear mixed-effects models, treating the blocks and plants as random variables and the treatments as fixed factors. To determine mean differences, we performed a Fisher's LSD comparison (p<0.05) with Bonferroni correction to ensure rigor in the comparison of means. The analyses were conducted using the statistical software R, version 3.6.0.
RESULTS AND DISCUSSION
Identifying an efficient and reproducible inoculation method that closely mimics the natural conditions of soil-borne pathogen infection is a crucial factor in studying pathosystems in the search for control methods and breeding processes (Ferreira et al., 2019).
The controlled inoculations in sweet granadilla and purple passion fruit plants resulted in the expected outcomes regarding infection levels at the evaluated concentrations of F. solani inoculum. In the year 2017, initial symptoms appeared at 33 days post-inoculation for both sweet granadilla and purple passion fruit across all concentrations.
For the year 2020, the onset of initial symptoms varied based on species and concentrations. In sweet granadilla plants, those inoculated with 2x106 conidia·g -1 showed symptoms after 28 days. The plants inoculated with 1x106 conidia·g -1 exhibited symptoms after 31 days, and those inoculated with 0.5x106 conidia·g -1 showed symptoms after 35 days.
In the case of purple passion fruit plants, symptoms became visible at 35 days for the higher concentrations (1x106, 2x106 conidia·gram-1), while the lower concentration (0.5x106 conidia·gram-1) led to symptoms appearing at 42 days.
Both species exhibited various stages of wilting, followed by chlorosis and subsequent defoliation associated with the pathogen infection process. We also observed symptoms at the base of the stem/root collar (canker), in some plants, and stem browning. At the root level, the most common symptoms were necrosis and growth reduction.
The experiments described in this study (2017 and 2020) demonstrated validity and reproducibility in establishing F. solani pathogenicity tests in sweet granadilla and purple passion fruit plants in the vegetative state under greenhouse conditions. Both plant species exhibited a higher infection pattern when inoculated with a greater concentration of pathogen conidia. The control group (without inoculation) did not exhibit symptoms related to the disease during the evaluation (data not showed).
Microbiological analyses of the substrate on PDA and Komada agar allowed for the isolation of Fusarium spp. colonies with morphological characteristics similar to those of the inoculated strain, confirming the presence of the pathogen in the substrate and roots of each treatment.
The three inoculum concentrations were significantly different (F = 5.21, p = 0.0235) according to the analysis of variance for the IS variable in sweet granadilla, while in purple passion fruit, no differences were observed among the three concentrations (F= 0.86, p=0.4481) (Table 3). These results suggest that using inoculum concentrations in the range of 0.5 to 2.0 × 106 F. solani conidia per gram of substrate is feasible. However, higher concentrations caused more damage to the plants. We observed significant differences among treatments when comparing the two experiments (2017 and 2020). In both experiments, there was a trend for higher disease severity index (SI) in response to higher concentrations of pathogen conidia for both Passiflora species (Figure 2).
Table 3 Severity indices of collar rot, based on visual symptom estimation in sweet granadilla inoculated with different concentrations of F. solani strains in the two combined experiments.
| Species | Treatment1 (conidia g-1) | SI (%)2 | LSD Fisher (Alfa = 0.05) |
|---|---|---|---|
| Sweet Granadilla | 0.5 × 106 | 50.35 ± 31.39 | b |
| Sweet Granadilla | 1 × 106 | 68.35 ± 24.54 | a |
| Sweet Granadilla | 2 × 106 | 73.01 ± 18.45 | a |
| Purple Passion Fruit | 0.5 × 106 | 62.80 ± 20.31 | a |
| Purple Passion Fruit | 1 × 106 | 72.87 ± 15.69 | a |
| Purple Passion Fruit | 2 × 106 | 62.45 ± 18.34 | a |
1 The inoculum of F. solani strains was produced in rice and mixed with moistened peat at field capacity before its application to sweet granadilla and purple passion fruit plants. 2 SI: Severity index: mean values from the two experiments (2017 and 2020). Means ± standard deviation with the same letter do not indicate significant differences in Fisher's LSD grouping test for means at a significance level of 5%.
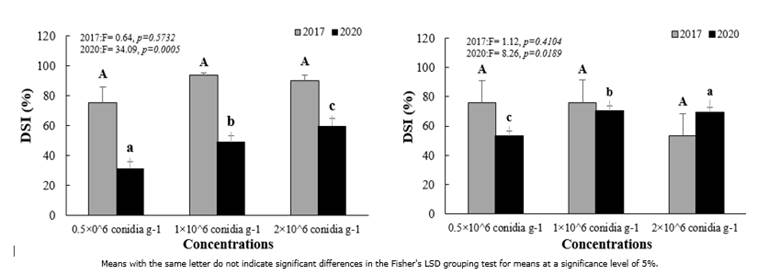
Figure 2 Comparison of severity indices, based on visual symptom estimation in sweet granadilla (left) and purple passion fruit (right) inoculated with different concentrations of F. solani strains in the two experiments (2017 and 2020).
When comparing the outcomes achieved in each species across the two experiments, we notice that the inoculations carried out in 2017 yielded higher severity indices and an earlier manifestation of symptoms. This could be attributed to the fact that these plants exhibited less developed root systems and smaller stem diameters. Fischer et al. (2005) reported similar findings. They evaluated plants at different phenological stages and noticed that younger plants were more susceptible because they had a narrower stem base or root neck. Plants inoculated in the 2020 experiment, developed characteristic disease symptoms over a longer period, with less impact on roots. These results could be explained by their more advanced phenological growth stages, compared to plants used in the 2017 experiment. However, symptoms on leaves and the base of the stem/root neck were very similar across experiments.
Studies conducted by Pereira et al. (2019) demonstrated the effectiveness of this type of field inoculation directly into the soil. They chose concentrations similar to those evaluated in this study and used maize flour colonized with F. oxysporum f.sp. passiflorae to assess resistance in passion fruit genotypes in the field. Thangavel et al. (2021) applied a similar inoculation method for the identification and initial report of F. oxysporum f. sp. passiflorae (Fop) in passion fruit plants in Northland, New Zealand. In this study, they emphasized the importance of not affecting the roots to achieve a natural disease development. Nonetheless, there is a need to establish standardized protocols based on an understanding of the pathogen's behavior. This constitutes an innovative and easily reproducible method, considering that most inoculation systems are carried out through invasive methods. These methods show greater success in inducing symptom development in plants under controlled conditions, but they fail to mimic the natural infection process triggered by F.sp. in plants (Ángel et al., 2018; Forero et al., 2015).
Additionally, in this research, we effectively implemented the scale designed by our research team in 2017, accompanied by a pictorial guide for assessing collar rot symptoms. This scale offers a higher level of detail compared to others reported in the literature, and it is specific to sweet granadilla and purple passion fruit collar rot (Tables 1 and Table 2).
In this study we also incorporate the measurement of the three types of indicative symptoms of collar rot: two types of primary or direct symptoms occurring at the base of the stem and the root collar (SBTCR), as well as in the roots (SR), and a set of above-ground symptoms (SH). These above-ground symptoms, while considered indirect or secondary in nature, are crucial for disease detection in practice. The two types of symptoms are causally related and, when used together, allow for an accurate diagnosis of collar rot in sweet granadilla. Consequently, they can be integrated, and transformed into a collar rot severity index (IS). This type of approach, as discussed by Fang et al. (2012), represents a necessary step to support epidemiological studies (Chiang et al., 2017a).
In line with the above, the method that we propose for inoculation and evaluation of collar rot response can be easily implemented as a protocol, through a set of activities depicted on a simple chronological format covering the distinct phases of the inoculation and response evaluation process. The plant material used for inoculations must be pathogen-free, possess a well-developed root system, exhibit genetic identity, and high physiological quality. Suitable plant material can be obtained from a commercial nursery or produced by the researcher and must be in a vegetative state (12 - 16 weeks). The inoculum should originate from a physiologically active strain previously characterized using conventional and molecular methods. Its viability must be confirmed in advance (Marostega et al., 2019), and it should be applied at a concentration of 1 × 106 F. solani conidia g-1 of peat, making sure to place it in contact with an intact plant stem base (it should have no prior damage).
Taking into consideration the analyses from the two experiments, the established inoculum concentrations, along with the developed protocol for its laboratory production and application to passion fruit seedlings, constitute a standardized system of controlled infection with F. solani that can be replicated for research purposes in passion fruits. These purposes include germplasm evaluation, epidemiological studies, and assessments of methods and products for collar rot control (Ferreira et al., 2019).
Likewise, it is necessary to verify through microbiological analyses that the irrigation water, substrate, and tools used for managing the plant material in the greenhouse are free from pathogens that could interfere with the proper development of the plant material or with the various processes of the plant-pathogen interaction resulting from the inoculation of Fsp. into sweet granadilla and purple passion fruit plants.
CONCLUSIONS
Our findings support the use of the established inoculum concentrations and the laboratory production protocol for infecting sweet granadilla and purple passion fruit plants with F. solani f.sp. passiflorae under controlled conditions. Additionally, we designed a scale for assessing primary symptoms at the base of the stem/root collar, as well as secondary symptoms on leaves and roots. This scale can be used for monitoring the disease in future research, including identifying sources of genetic resistance, understanding disease epidemiology, and evaluating methods for collar rot control.